рефераты конспекты курсовые дипломные лекции шпоры
- Раздел Иностранные языки
- /
- FURTHER ADVANCES IN THE FIELD OF RADIOACTIVITY
Реферат Курсовая Конспект
FURTHER ADVANCES IN THE FIELD OF RADIOACTIVITY
FURTHER ADVANCES IN THE FIELD OF RADIOACTIVITY - раздел Иностранные языки, THE PRE_ATOMIC AGE Their Work, Which Led To The Nobel Prize For Both (And Two Pr...
Their work, which led to the Nobel Prize for both (and two prizes for Marie, a rare honor), confirmed the cathode-ray research of British scientist J. J.Thomson, who had experimented with cathode rays and discovered that the rays were made up of positively charged particles that he called “corpuscles”, but which in fact were the electrons proposed by Stoney. Terminology not withstanding, Thomson deduced that the particles came from within the atoms of the electrodes in the cathode-ray tube, meaning that atoms were not indivisible or indestructible. Thomson’s announcement of his discovery and its meaning, in 1897, was the first major development: in the evolving view of the atom, and the work of the Curies not only supported but also augmented Thomson’s premise.
While the Curies continued with their work on radium and radioactivity (an initiative Marie continued after Pierre’s tragic death in a traffic accident), Thomson and his proteges at Britain’s Cavendish Laboratory also pursued their work. Thomson proposed a model of the atom that came to be known as the “plum pudding model”, in which, as he termed it “a number of negatively charged electrified corpuscles” were surrounded by a sphere of “uniform positive electrification”, or, as historian Richard Rhodes has suggested, “like raisins in a pudding”. It would fall to one of Thomson’s rapidly rising proteges, Ernest Rutherford, to test and ultimately prove another model. Between .1898 and 1911, as Rhodes would later eloquently phrase if, Rutherford “systematically dissected the atom”.
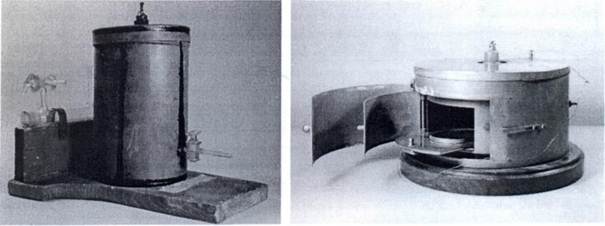
|
| Pierre Curie developed the ionization chamber to detect and measure radioactivity for his wife's experiments. A highly sensitive device, shown here with two examples, an ionization chamber contained a positive and a negative plate connected by an electrometer (which measures electrical currontsj. When a radioactive sample was placed in the chamber, air molecules broke down into positive and negative ion pairs, which allowed them to carry electrical current. As the negative ions migrated to the positive plate, and the positive ions migrated to the negative plate, the electrical current flowed through the electrometer, which Curie could then read. The strength of the current was a direct result of the level of radiation. (Bridgeman Art Library) |
As Rutherford studied the rays that the Curies had identified, he determined, in 1899, that there were two types of rays. The first, a high-energy burst of easily-blocked and absorbed radiation, which lie called alpha radiation, and another, more penetrating type that he-called beta radiation. A year later, in 1900, a third, even more penetrating type was identified and named gamma radiation by its discoverer, Paul Villard of France. By examining a radioactive gas emitted by thorium, Rutherford and his assistant, Frederick Soddy, were able to calculate the decay of radioactive materials, and by noting the time it took for half of a sample to do so, determined what is now known as the “half-life” of radioactive elements and isotopes.
As the secrets of the atom were being probed, the concept that the atom possessed a tremendous potential for untapped energy became clear. The work of the Curies had shown how heat was generated by radium, and visitors to Marie Curie s lab could visibly see the results in radiation burns on the scientist’s hands. In 1905, German physicist Albert Einstein proposed, as part of his theory of special relativity, mass-energy equivalence, a now-famous formula expressed as E=mc. While it would take years for science to catch up to Einstein’s theory and prove its validity, it suggested tremendous potential for the atom. The formula would later be used during the development of the atomic bomb to determine the energy available in an atomic nucleus, and hence the energy released in a nuclear reaction.
In 1911, Rutherford’s “dissection” of the atom determined that the once seemingly impenetrable atom was in feet a series of electrons that orbited a nucleus in space. Shooting rays through a thin piece of gold foil, Rutherford and his assistants noticed that not all energy passed through the foil. He wrote, “considering the evidence as a whole, it seems simplest to suppose that the atom contains a central charge distributed through a very small volume, and that the large single deflexions are due to the central charge as a whole, and not to its constituents”. The “concentrated central charge”, (later termed the nucleus) set the stage for further refinement, which came quickly.

| Sir Joseph John Thomson (1856-1940) of Trinity College, Cambridge, discovered the electron in his cathode-ray experiments. His discovery, announced in 1897, was the first major development in the evolving view of the atom. Thomson received the Nobel Prize in Physics in 1906. (Bridgeman Art Library) |

| Shown here in their Paris laboratory, the husband and wife team of Pierre [1859-1906] and Marts Shlodowska Curie (186? 1934) worked together to discern the secrets of the atom, distinguishing alpha, beta and gamma radiation. Following Pierre's tragic death, Madame Curie continued her research, earning the No'ael Prize in Chemistry in 1911 for the discovery of polonium and radium, and for the isolation of pure radium. (Bridge-man Art Library) |
– Конец работы –
Эта тема принадлежит разделу:
THE PRE_ATOMIC AGE
TEXT A... THE PRE ATOMIC AGE...
Если Вам нужно дополнительный материал на эту тему, или Вы не нашли то, что искали, рекомендуем воспользоваться поиском по нашей базе работ: FURTHER ADVANCES IN THE FIELD OF RADIOACTIVITY
Что будем делать с полученным материалом:
Если этот материал оказался полезным ля Вас, Вы можете сохранить его на свою страничку в социальных сетях:
| Твитнуть |
Хотите получать на электронную почту самые свежие новости?






Новости и инфо для студентов