рефераты конспекты курсовые дипломные лекции шпоры
- Раздел Иностранные языки
- /
- FURTHER DEVELOPMENT OF ATOMIC THEORY IN THE 19th CENTURY
Реферат Курсовая Конспект
FURTHER DEVELOPMENT OF ATOMIC THEORY IN THE 19th CENTURY
FURTHER DEVELOPMENT OF ATOMIC THEORY IN THE 19th CENTURY - раздел Иностранные языки, THE PRE_ATOMIC AGE By The Early 19Th Century, Atomism Had Re-Ent...
By the early 19th century, atomism had re-entered scientific thought, particularly chemistry, where it strongly influenced British philosopher and mathematician John Dalton (1766-1844). As early as 1803, Dalton, after experimenting with gases, put forward a chemical atomic theory that proposed a more sophisticated model than Democritus or Gassendi. Dalton’s theory, the basis of modern atomic thinking despite some error, was that

| Wilheim Conrad Rontgen (1845-1923).R6ntgen's 1895 experiments with the flow of electrical currents through gas of an extremely low pressure resulted in his discovery of a new type of ray that permeated most objects. He called the new phenomenon “X-rays” because their nature was unknown to him. Rontgen’s work inspired the work of French scientist Marie Curie, and led to his receiving the Nobel Prize in Physics in 1921. (Bridgeman Art Library) |
atoms combined to form chemical elements. He believed that the atoms in any one element were identical in their masses (an error), but atoms of different elements had different masses. He also believed that atoms could only combine in small, whole-numbered rations (1:1, 2:3, etc.). Dalton based his argument that atoms of different elements had different weights by experimenting with elements to obtain relative particle weight. He was the first scientist to do so. His Table of the Elements, giving them standard symbols, was another great Dalton achievement. Dalton’s laboratory work modernized “atomism”, and inspired later generations of chemists and other scientists to continue to probe atomic theory. One aspect of that theory, unchanged since antiquity, would be the arena where the greatest breakthroughs would come. That was the concept that atoms were unchangeable, and indestructible. Dalton continued the ancient argument, noting, “we might as well attempt to introduce a new planet into the solar system, or to annihilate one already in existence, as to create or destroy a particle of hydrogen.” Future researchers, working with new technology and concepts, wouldprovide the tools to do so.
As the end of the 19th century approached, the Marquis of Salisbury, Robert Cecil, speaking to the British Association for the Advancement of Science in 1894, listed the “unfinished business of science” and posed questions about the atom, namely “whether it is a movement, or a thing, or a vortex, or a point having inertia, whether there is any limit to its divisibility, and if so, how that limit is imposed, whether the long list of elements is final, or whether any of them have any common origin, all these questions remain surrounded by a darkness as profound as ever.”
The first light to illuminate that darkness came from the cathode-ray tube. The cathode-ray tube was the initial child of the development in 1855 of a mercury pump by Heinrich Geissler, which produced vacuum tubes of high quality. Others, such as Sir William Crookes, discovered that when one end of a tube was capped with metal, and a battery was hooked up to them, the airless space inside the tubes glowed, passing from the negative plate - the cathode - to the positive plate, or the anode. When the cathode and anode were placed inside the middle of a tube, and the end of the tube was closed off with glass, the glow would become a beam, or a ray.
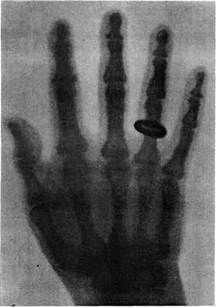
| Rontgen captured a series of early images made by X-rays on photographic plates, including one of his wife’s hand wearing her ring, which he made on January 25, 1896. Known as roentgenograms, the plates were visual proof of a hitherto invisible phenomenon. (Bridgeman Art Library) |
German and British researchers, working with cathode-ray tubes, learned more about the rays between 1858 and 1894. The rays bend when under the influence of a magnet; otherwise they travel in straight lines. They are formed of particles of some sort of matter, which have a charge. In 1874, James Johnstone Stoney, in calculating the charge, suggested that the unit of charge be called an “electrine”. In 1891, he changed the name to “electron”.
Then, in 1895, the German physicist Wilhelm Conrad Rontgen, experimenting with covering tubes with screens to determine the nature of the fluorescent light emanating from them, made an amazing discovery. Even when blocked by cardboard, the tube produced a glow on a nearby screen of chemical-coated black paper. Passing his hand in front of the tube to block the glow, Rontgen discoveredthat it did not completely block it - and, in the dim light, he could see his bones through the flesh of his hand. A new type of ray - not light - was produced by the cathode-ray tube, and the name Rontgen gave it - the X-ray - stuck.
– Конец работы –
Эта тема принадлежит разделу:
THE PRE_ATOMIC AGE
TEXT A... THE PRE ATOMIC AGE...
Если Вам нужно дополнительный материал на эту тему, или Вы не нашли то, что искали, рекомендуем воспользоваться поиском по нашей базе работ: FURTHER DEVELOPMENT OF ATOMIC THEORY IN THE 19th CENTURY
Что будем делать с полученным материалом:
Если этот материал оказался полезным ля Вас, Вы можете сохранить его на свою страничку в социальных сетях:
| Твитнуть |
Хотите получать на электронную почту самые свежие новости?






Новости и инфо для студентов