рефераты конспекты курсовые дипломные лекции шпоры
- Раздел Образование
- /
- Part One THE PREDICATE
Реферат Курсовая Конспект
Part One THE PREDICATE
Part One THE PREDICATE - раздел Образование, Part One The Predicate Lesson 1 Грамматика...
Part One THE PREDICATE
Lesson 1
ГРАММАТИКА: Определение сказуемого в предложении. Простое сказуемое в действительном залоге (Indefinite). Употребление платла will для выражения регулярности действия в настоящем времени» глаголов used to н would—для выражения повторностн в прошлом. Функции глагола do.
Section I
Ex. 1. Pronounce the following words:
|
b) hypothesis [hai'poOosis], theory I'Oion], law [Id:], subject [sob'dsekt], agree [o'gri:], atom fastom], atomic [o'tomik], universe [ ju:niv3:s], example [tg'za:mpl], chemist ['kemist]
Ex. 2. Read the following words and say what Russian words help to understand their meaning:
theory, hypothesis, correlate, test, deduction, result, experiment, atom, nature, crystal, substance, regular, interpret, systematic, argument, structure
Ex. 3. Pay attention to the following way of word-building:
основа слова + -л/ —» прилагательное
international» central, theoretical, hypothetical, experimental, industrial, formal, natural
прилагательное + -ly -> наречие
usually, readily, quickly, slowly, usefully, highly, easily, really, experimentally
основа слова + -fcf [ist] -> существительное, прилагательное communist, scientist, chemist, socialist, capitalist, naturalist, physicist, economist
Which of these words may be nouns and adjectives?
Ex. 4. What part of speech docs each of these abbreviations stand for: a, advy attry cj9 int, n> пит,part,prcp>prony v? What part of speech do the following words belong to? Write corresponding abbreviations for them.
theory» find, further, it, and, of, if, such, usually, about, part, represent, that, we, various, between, value, pure, crystal, atom, other, word, two, knowledge, by, thus, but, explain
Ex. 5. Define what parts of speech the italicized words belong to.
1. The girl's face was beautiful. 2. Many difficulties face us when we study. 3. Experimentally means during the experiment. 4. There are various means of getting substances. 5. Chemistry is a very interesting subject.
6. When we want to learn more about a substance, we subject it to study.
7. When you arc not sure that your result is right, check it up. 8. During the check he found a bad mistake. 9. This part of the work is the most difficult one. 10. They parted in 1985 when she went to Moscow. 11. In crystals the atoms arc in a regular order. 12. The director ordered him to come at once. 13. We measure weight by grams, kilograms, tons, etc. 14. A kilogram is a weight measure.
Text 1 A Hypotheses, Theories and Laws
When we find that an idea explains or correlates a number of facts, we call this idea a hypothesis. We can subject it to further tests and to experimental checking of deductions. If the hypothesis continues to agree with the results of experiment, we call it a theory or a law.
A theory, such as the atomic theory, usually involves some idea about the nature of some part of the Universe, a law represents a summarizing statement about observed experimental facts. For example, there is a law of the constancy of the angles between the faces of crystals. The law states that whenever we measure the angles between corresponding faces of various crystals of a pure substancc, they will have the same value. It docs not explain the fact. We find an explanation of the fact in the atomic theory of crystals, the theory that in crystals the atoms are in a regular order.
Chemists and other scientists use the word "theory" in two different senses. The first meaning of the word is the meaning described above — namely, a hypothesis that has been verified. The second use of the word "theory" is to represent a systematic body of knowledge, compounded of facts, laws, theories, deductive arguments and so on.
Thus, by the atomic theory we mean not only the idea that substances consist of atoms, but also all the facts about substances that can be explained and interpreted in terms of atoms and the arguments that explain the properties of substances in terms of their atomic structure.
Words and Word-Combinations to Be Memorized
also, angle, atom, atomic, body, check, consist of, correspond, crystal, example, for example, experiment, fact, further, hypothesis (hypotheses), idea, involve, in terms of, law, measure, namely, not only... but, observe, order, represent, result, same, state, statement, subject, such, test, theory, thus, universe, usually, various, verify, whenever
Ex. 6. Give the Russian equivalents for the following:
find, a number of facts, idea, subject to, experimental checking, agree with smth. (smb.), such as, the atomic theory, usually, involve, law, represent, for example, the faces of crystals, state, whenever, a pure substance, the same value, explain, in a regular order, in a sense, the meaning of the word, above, namely, verify a hypothesis, a body of knowledge, and so on, thus, not only... but, in terms of
Ex. 7. Give the English equivalents for the following:
объяснить факт, ряд экспериментов, подвергуть гипотезу проверке, экспериментальное подтверждение, согласовываться с результатами, называть законом, атомная теория, такой как, обычно, включать в себя, вселенная, утверждение, например, угол между гранями кристалла, измерять, различные вещества, то же самое значение, описанный выше, и так далее, таким образом, не только... но и, с точки зрения
Ex. 8. Fill in the blanks with prepositions where necessary.
of, to, with, for, in, by, in terms of
1. If an idea explains or correlates a number ... facts, we call it a hypothesis. 2. Scientists subject the idea ... experimental chccking. 3. If the hypothesis agrees ... the results ... experiment, we call it... a theory or ... a law. 4. ... example, everybody knows the periodic law. 5. The explanation ... the fact is... the atomic theory. 6.... crystals the atoms are
... a regular order. 7_ the atomic theory we mean that substances consist
... atoms. 8. Scientists explain facts ... atoms and their structure.
Ex. 9. Practise the numerals.
a) 1,2,3,4,5,6, 7, 8,9,10,11, 12,100, 1,000
b) Суффикс -teen образует числительные от 13 до 19, всепаа ударный. Иногда при добавлении суффикса происходят изменения основы слова.
Model: 14 — fourteen Исключения: 13 — thirteen
15 — fiyicen
13,14,15,16,17,18, 19
c) Суффикс -ty служит для образования десятков, неударный.
Model: 60 — sixty Исключения: 20 — Amity
30 — thirty 40 — forty 50 — fifty
20,30,40,50,60,70,80,90
d) Сложные числительные.
Model: 21 —twenty-one
2432 — two thousand four hundred and thirty-two
23,46,34,27,93,62, 87, 55,71, 953,281,450, 374,799, 543, 827, 665,1946,5812,2432,7250,1240,9824,7561
e) Суффикс -th служит для образования порядковых числительных.
Model: four — fourth Исключения: I -й — first
2- й — second
3- й —third
5-fi — fiflh
5-й, 6-й, 7-й, 8-й, 9-й, 10-й; 56-й, 73-й, 89-й, 94-й, 25-й, 63-й; 914-й, 852-й, 263-й, 427-й, 791-й; 2406-й, 1433-й, 5813-й, 4743-й, 3585-й
0 Числительные, обозначающие даты.
Model: до 2000-го года: 1946 — nineteen forty-six; 2000-й год: two thousand; после 2001-го года: 2004 — two thousand and four
1945,1917,1812,1799,1242,1961,1957,1825,2001,2003,2005
Ex. 10. a) Read the following forms of the verbs; mind different ways of reading -5 and -cd.
explains, correlates, continues, calls, involves, states, uses, verifies, means; called, subjected, checked, involved, represented, observed, corresponded, used, described, consisted
b) Give the three forms of the following verbs:
be, have, find, do, mean, give
с) Translate the sentences into Russian.
I. A theory usually involves some idea about the nature of some part of the Universe. 2. The periodic law represents a summarizing statement about the properties of elements. 3. The scientists verified die hypothesis and it became a theory. 4. A law will not explain die fact, it only states the fact. 5. Early scientists would use the word 'theory" in two different senses, now we do the same. 6. A hypothesis usually explains or correlates a number of facts. 7. Now we are speaking about hypotheses, theories and laws. 8. The atomic theory explains and interprets die facts in terms of atoms. 9. A good theory will live a long life. 10. Will you tell us what you know about a hypothesis? 11. What do we call a law? 12. Early scientists did not think of atoms. 13. Now the atomic theory is more popular than it used to be. 14. Before the nineteenth ccntuiy chemists would not think about families of elements. IS. The periodic law docs help chemists in their work. 16. Have you translated the sentences about the atomic theory? 17. By the atomic theory of crystals the atoms in them will be in a regular order.
Ex. 11. Translate the following sentences into English:
1. Закон — это такое утверждение, которое суммирует наблюдаемые экспериментальные факты. 2. Теория даст экспериментально подтвержденное объяснение фактов. 3. Вы поняли, что такое гипотеза?— Да, это шея, которая объясняет или сопоставляет ряд фактов. 4. Когда эксперименты действительно подтверждают какую-то гипотезу, мы обычно называем ее теорией. 5. Ученые знают много гипотез, которые стали теориями или законами, б. Химики широко пользуются атомной теорией. 7. Древние ученые думали о структуре веществ. 8. Мы объясняем свойства веществ с точки зрения их атомной структуры.
Ex. 12. Make up questions to the italicized parts of the sentences.
1. Chemists use the word theory in two different senses (3)[1]. 2. We usually subject a hypothesis to further tests (2). 3. Every science has its laws (2).
Ex. 13. Answer the following questions:
1. What is a hypothesis? 2. Do you know what a law is? 3. Do you know any laws? 4. What is a theory? 5. What theories do chemists use in their work? 6. What do wc mean by the atomic theory? 7. When did you hear about the atomic theory for the first time?
Section II
Упр. 1. Прочтите заглавие текста 1 В и скажите, о чем, по вашему мнению, в нем может идти речь.
Упр. 2. Назовите значения следующих интернациональных слов:
modern, accumulate, revolutionary, status, molecule, real, electronic, collcction, principle, arrange, system
Упр. 3. Определите значения выделенных слов по контексту.
1. Dalton developed his theory between 1801 and 1808.2. The atomic hypothesis appeared long ago. 3. A hypothesis is an assumption which explains or correlates the facts. 4. A theory concerning the structure of substances is the atomic theory. 5. The periodic law summarizes the properties of elements. 6. A verified idea is no longer a hypothesis. 7. Students want to have deep knowledge in chemistry. 8. Chemists study the behaviour of substances. 9. By Dalton's theory an atom was the smallest particle.
Слова к тексту:
direct evidence — прямое доказательство; existence — существование; predict—предсказывать; indivisible—неделимый; size—размер; weight— вес; shape — форма; test-tube — пробирка; breakthrough — проникновение, прорыв; unrelated — несвязанный; mature—зрелый
Text 1 В fc
I Прочтите текст про себя (контрольное время чтения — 7 минут).
Dalton's Atomic Theory
i One of the foundations of modern chemistry is Dalton *s atomic theory {developed between 1801 and 1808. When the atomic hypothesis appeared, fUierc was no direct evidence of the existence of atoms. But as time passed, Scientists found that the assumption of their existence and other assumptions ^concerning their properties and behaviour explain more and more of the accumulating cxpcrimantally determined facts of chemistry, and also ^predict other facts successfully. The hypothesis, therefore, gained the status <of a theory.
There were revolutionary changes in chemistry in the last fifty years. Chemists found that atoms arc not indivisible particles as Dalton thought, but consist of much smaller particles which form the structure of atoms. They established their sizes, weights and the arrangement of their parts with high accuracy, as well as the sizes and shapes of molecules and the internal structure of crystals. As a result, atoms and molecules arc now as real as test-tubes. Chemistry has achieved not only a new look, but a major breakthrough to a deeper level of understanding. It is now possible to explain most of the properties of elements and their compounds in terms of their electronic, atomic and molecular structures. It is also possible to predict new properties successfully. Chemistry is no longer a collcction of more or less unrelated facts, but a mature science founded on scientific principles wliich arrange facts in an orderly system.
Упр. 4. Скажите, совпали ли паши предположения с содержанием текста: а) полностью; б) частично; в) совсем не совпали.
Упр. 5. Как иначе можно было бы озаглавить этот текст? На сколько тематических частей его можно разделить? Укажите начало и конец каждой части.
Упр. 6. Скажите, что говорится в тексте:
1) о том, как гипотеза об атомном строении вещества стала теорией; 2) об изменении химии как науки; 3) о том, что нужно делать, основываясь иа современном уровне знания химии; 4) о том, как характеризуется современная химия.
Упр. 7. Найдите в тексте предложения, в которых говорится о том, как изменилась химия со времен Дальтона, и переведите их на русский язык.
Section Ш
Ex. 1. Ask your follow students to do or not to do the following:
1) go to the blackboard; 2) open the door; 3) write the words on the blackboard; 4) clean the blackboard; 5) read the text aloud; 6) translate the sentence; 7) ask you about something
Ex. 2. a) Check up if you remember the following expressions:
Will you do it? Would you tell me? Don't you mind? Shall 1 read?
b) Translate the sentences into English using the above expressions.
1. Вы не возражаете, если я задам вам вопрос? 2. Пожалуйста, скажите мне, что такое закон. 3. Мне отвечать? 4. Не скажете ли вы, когда Дальтон разработал свою атомную теорию? 5. Вы ничего ис имеете против, если я буду читать вслух? 6. Текст читать про себя? 7. Пожалуйста, объясните, как изменилась химия. 8. Мне сделать это сейчас, или это домашнее задание? 9. Не читайте этот текст без словаря: он очень трудный.
Ex. 3. Make up sentences with the following words and expressions:
1) fact, idea, explain, hypothesis, correlate; 2) law, represent, statement, about, facts, observe; 3) chemist, atomic, long ago, know, theory; 4) in the latest years, change, chemistry, revolutionary; 5) science, principle, scientific, chemistry
Ex. 4. Give detailed answers to the following questions:
1. What is the difference between a theory, a law and a hypothesis? 2. What are the two meanings of the word "theory"? 3. What laws in chemistry do you know? 4. How did Dalton's atomic hypothesis gain the status of a theory?
Ex. 5. Discuss the following topics:
1. Dalton's Atomic Theory.
2. The Changes in the Atomic Theory since Dalton's Time.
3. Chemistry as a Science.
4. What Scientists Know about the Structure of Substances.
DO YOU KNOW THAT...
M. V. Lomonosov (1711-1765) contributed greatly to the theoretical development of physics, astronomy, geography, mineralogy and other sciences. He worked out his own corpuscular theory and its chemical application. His corpuscular theory was the basis for the future development of the atomic theory. He is the founder of Russian physico- chemical science.
Lesson 2
ГРАММ АТИ К А: Простое сказуемое в действительном залоге (Continuous, Perfect).
Scction I
Ex. 1. Pronounce the following words.
| e | BO | he, we, be, genius |
| e | [c] | let, help, next, effort, desk, get, left, lesson, member |
| cr, cr + согласная | [з:] | her, serve, inert, certain, perfect, observe, service, determine |
| cr + гласная | [1Э] | here, period, mere |
| car | [к») | hear, near |
| [з:] | learn, early, earth, research | |
| ca cc | BO | teach, dream, clean, each, east, speak, leave, mean, read, sea, feature see, need, meet, green, agree, three, street, degree |
b) chemical ['kcmikol], great [greit], nature ['ncitjo], allow [o'lao], idea [ai'dio], experiment [iks'penmont], bear [bed], colleague [#koli:g], uranium [ju'reinjamj, nuclear fnjuikho]
Ex. 2. Read the following words and say what Russian words help to understand their meanings:
periodic, system, clement, history, genius, characteristic, idea, calculation, modern, colleague, pedagogical, meteorology
Ex. 3. Pay attention to the following way of word-building:
основа глагола + -tion [Jn] -» существительное
consideration, contribution, direction, prediction, calculation, foundation, intensification
основа глагола + -er (ч>г) [о] —> существительное
teacher, researcher, founder, discoverer, writer, worker, explorer, observer,
learner, receiver, speaker, visitor
основа слова + -ic [lk] —» нрнлагателыюе
periodic, characteristic, specific, energetic, economic, scientific, historic
Ex. 4. Give the initial forms of the following words:
served, greatest, making, shows, placcd, properties, researches, ideas, hypotheses, theories, laid, closcd, villages, areas, said, tried
Ex. 5. Define what parts of speech the italicized words belong to:
1. The periodic system of the elements was the greatest contribution to chcmistry. 2. It was really the work of a genius. 3. It adds much to the present knowledge. 4. The element No. 101 bears the name of Mcndclcycv. 5. The periodic system is the basis of modern teaching on substances.
Text 2 A The World's Greatest Chemist
The periodic system of the chemical elements by Mcndclcycv has long since served as the greatest history-making contribution to the study of nature. As any work of genius it shows two characteristic features: it adds more to the present knowledge, and it fruitfully develops along different directions in future.
It allowed to predict in advance the existence and properties of yet undiscovered elements. Many outstanding researchers owe to it, to a considerable degree, the ideas of their experiments, calculations, hypotheses and theories. Take, for example, the German Otto Hahn, who discovered the fission of the uranium nucleus. Or the American Glenn Scaborg who led a group of researchers that obtained, in laboratory conditions, a number of elements, including mcndclcvium, named in honour of Mcndclcycv. That element bears die name of the great Russian scientist not only because Mcndclcycv laid the foundation of the modern science of atom, but also bccausc he drew his colleagues' special attention to uranium (No. 92), which at the time had closcd his periodic tabic. A long train of transuraniums followed the once "final" uranium.
"The Mcndclcycv system has served for almost 100 years as a key to discovering new elements," Scaborg wrote in 1955. It has retained its key capacity until now.
To commcmoratc Mcndclcycv himself, the Soviet researchers named many newly discovered things on the earth or in the outer space after him: a crater on the "back" side of the Moon, an underwater ridge in the Arctic Ocean and the mineral mcndclcyevitc. Villages, streets and establishments such as the Moscow Institute of Chemical Technology, the Tobolsk Pedagogical Institute, the All-Russian Institute of Meteorology, the Museum of the St. Petersburg University building (where the scientist lived), the All-Russian Chemical Society, etc. have got Mcndeleycv's name.
Mcndclcycv, the explorer of nature, has found real immortality in his pasting heritage. The periodic system hasn't crumbled with time; on the contrary, its structure has expanded. At present it is the basis of modern teaching on substances, the structure of matter, atoms and nuclear energy.
"The greatest chemist of the world" — this is Mcndeleycv's fame among modern chemists. Yes, he, the founder of modern chemistry and, to a large degree, of modern physics, considered physical chemistry his main subject, while he successfully dealt with problems in different areas, from mathematics and astronomy to meteorology, from philosophy to economics, from technology to art. "He has penetrated everywhere," the great Russian poet Alexander Blok once said.
Mcndeleycv's notes on "three services to the Motherland" arc quite interesting. He places work as an explorer of nature at the first place. He devoted himself to it. He tried to make his experimental and theoretical results serve society. He also devoted much of his effort to teaching, to the spread of knowledge. Finally, the third important task in Mcndeleycv's life was to do his best for the economic and industrial progress of Russia.
Mcndeleycv's dreams have come true. As long as seventy years ago the British magazine Nature (of February 24, 1934) wrote that in Russia scientists like Mcndclcycv are valued and their works help to intensify the development of science, technology and industry.
Words and Word-Combinations to Be Memorized
almost, attention, basis, bear, calculation, characteristic, chemical, chemist, chcmistry, contribution, develop, devote, direction, draw attention, element, existence, feature, found, knowledge, lay the foundation, modern, nature, outstanding, period, periodic, predict, present, at present, property, rcscarchcr, revolution, scientist, special, structure, subject, substancc, system
Ex. 6. Give the Russian equivalents for the following:
the greatest chemist, the periodic system of the elements, any work, characteristic features, to predict in advance, undiscovered elements, an outstanding researcher, lay die foundation of smth., modem science, special attention, nuclear energy, deal with smth., devote oneself to, do one's best, chemical society
Ex. 7. Give the English equivalents for the following:
изучение природы, свойства элементов, носить имя кого-л., великий русский химик, современная химия, обратить внимание на что-л., назвать чьим-л. именем, всероссийский институт, наоборот, в настоящее время, физическая химия, основной предмет, мечты осуществились
Ex. 8. Fill in the blanks with prepositions where ncccssary.
of опу m, to> after
1. The periodic system ... the elements allowed to predict ... the existence and properties ... some elements. 2. D. I. Mcndclcycv laid the foundation ... the modern science ... atom. 3. D. I. Mcndclcycv drew attention ... chemists ... uranium. 4. The Soviet researchers named many newly-discovered things... the earth and... the outer space... Mcndclcycv. 5.... present the periodic law is the basis... modern teaching... substances.
6. D. I. Mcndclcycv considered physical chemistry ... his main subject.
7. He devoted much ... his efforts ... teaching.
Ex. 9. Translate the sentences into Russian. Pay attention to the construction there be.
1. There arc 9 elements in Group I. 2. There is only 1 electron in the hydrogen atom. 3. There arc many things on the earth which arc named after Mcndclcycv. 4. There arc many institutes in Russia which have got Mcndclcycv's name. 5. There arc some elements which don't exist in nature, scientists obtained them in laboratory. 6. There arc some elements in Group I that arc very active. 7. There arc some elements which arc not active. In what group arc they? 8. There arc many features in which elements differ radically. 9. Originally there were fewer elements in the periodic table.
Ex. 10. Translate the sentences into Russian.
I. Since its discovery the periodic system of the chemical elements has long served and is still serving as the greatest contribution to the study of nature. 2. This century has seen great changes in science and the life of people. 3. The ideas of many outstanding researchers originate from the periodic law. 4. The All-Russian Chemical Society bears the name of Mcndclcycv. 5. The structure of the periodic system has expanded to a considerable degree. 6. A person of wide interests, Mcndclcycv successfully dealt with problems in mathematics, astronomy, meteorology, philosophy, economics and art. 7. He placed work as an explorer of nature at the first place. 8. Mcndclcycv tried to do his best for the economic and industrial progress of Russia. 9. Works of outstanding chemists help to intensify the development of science, technology and industry. 10. A Russian name appeared in 1964 on the honorary board of scicncc at Bridgeport University, USA,—Mcndclcycv's name appeared in the list of the greatest geniuses — Euclid, Archimedes, Copcmicus, Galileo, Newton and Lavoisier. 11. Mcn- dclcycv published his periodic system in 1869. 12. Mcndcleyev's law helped the American Glenn Seaborg who led a group of researchers to obtain a number of elements, including mcndclcvium, in laboratory conditions. 13. The laboratory led by Academician Gcorg Flyorov has been the cradle of many transuraniums. 14. Mcndclcycv had been active in founding the Russian Chcmical Society, which held its first meeting on November 6, 1868. IS. The predictions did not seem surprising; the successive discoveries of gallium (1874), scandium (1879), and germanium (1885) followed. 16. It is a remarkable fact that Mcndclcycv actually spent only a few years in developing the periodic table, and then went on to other work.
Ex. 11. Translate the sentences into English.
1. Периодическая система элементов Д. И. Менделеева стала известна в 1869 году. 2. Она позволила заранее предсказать существование и свойства нескольких элементов. 3. Менделевии, один из трансурановых элементов, носит имя ученого, который привлек внимание коллег к урану. 4. Д. И. Менделеев — основатель современной химии и, в значительной степени, современной физики. 5. Он посвятил себя изучению природы. 6. Всю свою жизнь Д. И. Менделеев делал все возможное для прогресса России в области науки и экономики. 7. Его закон заложил основу для современной пауки об атоме. 8. В настоящее время периодическая таблица сильно отличается от периодической таблицы 1869 года. 9. Каждый период таблицы Менделеева содержит определенное число элементов. 10. Например, в первом периоде — только два элемента. 11. До Менделеева были попытки расставить элементы в некотором порядке.
Ex. 12. Make up questions to the italicized parts of the sentences.
I. Mcndclcycv is one of the greatest chemists (2). 2. He published his periodic system in 1869 (3). 3. Mcndclcycv predicted some properties of undiscovered elements (2).
Ex. 13. Answer the following questions:
1. Why is the periodic system by Mcndclcycv valued so much? 2. Why docs the clement No. 101 bear Mcndeleycv's name? 3. Has the periodic table changed with time? In what way has it changed? 4. What information is it possible to get from the periodic table of elements? 5. Was D. I. Mcndclcycv a man of wide interests? Prove it. 6. What docs the periodic law state?
Scction II
Упр. 1. Прочтите заглавие текста 2 В и скажите, о чем, по вашему мнению, в нем может идти речь?
Упр. 2. Назовите значения следующих интернациональных слов:
examination, the Julian calendar, rudimentary, arrangement, vertical, horizontal, transpose, operate, bureau, visit
Упр. 3. Определите значения выделенных слов по контексту.
1. Mcndclcycv set down his first ideas at breakfast. 2. He received a note about a visit to a cheese factory, but he cancelled the visit and worked on. 3. His mother operated a glass factory to keep the wolf from the door. 4. His mother headed for Moscow. 5. She wanted to place her son in the University of Moscow, but he was refused. 6. The government retired Mcndclcycv from flic University of St. Petersburg. 7. He bccamc head of the Bureau of Weights and Measures in 1893 and held that post until his death.
Слова к тексту:
devise — придумывать; brainwave — осенившая кого-л. идея; card — карточка; j uggle—манипулировать; satisfy—удовлетворять; nap—дремота, короткий сон; go blind — ослепнуть; lease — арендовать; clash—столкновение
Text 2 В
Прочтите текст про себя (контрольное время чтения — 7 минут).
The Mendeleyev Story
In a long examination of the periodic table of the elements, the New Scientist for March 7 tells briefly how Dmitry Ivanovich Mendeleyev (1834-1907) devised it. The Russian scientist had his brainwave, says author John Emslcy, on March 1, 1869, but, because the Russians were still using the Julian calendar, it was February 17 in St. Petersburg, where Mendeleyev lived then.
Mendeleyev set down his first ideas at breakfast, on a note he had received about a visit to a cheese factory that day. He cancelled the visit and worked on. He drew up several rudimentary tables, Emslcy says, and then made 63 cards, one for each of the known elements. On each card he put the properties of the clement that he thought most important. He juggled the cards until he had an arrangement that satisfied him, wrote it down, and went to bed. He awoke from his nap with the idea that he should arrange the elements in vertical rather than horizontal groups and transposed them accordingly.
Mendeleyev was bom in Siberia and was the last of 17 children. In the year of his birth his father went blind, and his mother leased and operated a glass factory to keep the wolf from the door. His father died in 1847, and the glass factory burned down in 1848. With this his mother headed for Moscow with Dmitry and his sister. Her idea was to place the son in the University of Moscow, but he was refused because he had been bom in
Siberia. The same thing happened in St. Petersburg, but Dmitry's mother, ten weeks before death got him into die Pedagogic Institute there.
The government retired Mcndclcycv from the University of St. Pc- tcrsburg in 1890 for his political activities. He was made head of the Bureau of Weights and Measures in 1893 and held that post until his death.
Упр. 4. Скажите, что вы узнали из текста 2В; разделите его на тематические части и озаглавьте их.
Упр. 5. Скажите, что говорится в тексте:
1) о семье Д. И. Менделеева; 2) о том, как и куда он поступал учиться; 3) о том, как Д. И. Менделеев пришел к мысли о расположении элементов в таблице; 4) о том, чем занимался Д. И. Менделеев в последние годы своей жизни.
Ex. 6. Найдите в тексте и переведите на русский язык предложения, в которых говорится о том, как Д. И. Менделеев воплотил свою идею об упорядоченном расположении элементов.
Section III
Ex. 1. Think оГ the situations when the following expressions may be used:
to my mind; I don't think so; I agree with you; it's (quite) right; it's not right; sorry, you are wrong
Ex. 2. Express your agreement or disagreement with the following statements. Use expressions from exercise 1.
1. Mcndclcycv was the only scientist who tried to arrange die elements. 2. A lot of new elements were predicted with the help of the periodic table. 3. To commemorate Mcndclcycv, the Soviet researchers named many newly discovered things after him. 4. The periodic system didn't change with time. 5. Mendeleyev was interested only in chemistry. 6. Mcndeleycv's family was small. 7. Mendeleyev got his education in St. Petersburg.
Ex. 3. Make up sentences or short stories with the following words and expressions:
1) be bom, children, year, father, mother, 2) head, hold the post, bureau; 3) periodic system, serve, contribution, study; 4) the element, bear the name; 5) devote, teaching, development, economy, progress
Ex. 4. Give detailed answers to the following questions:
| БИБЛИОТЕКА ВГУ |
1. How did D. I. Mendeleyev devise his periodic table? 2. What is the importance of the periodic system of the elements? 3. Why do people call Mcndclcycv "the greatest chemist of the wnrld"? 4 What /jmnmc of
Mcndclcycv have come true nowadays?
Ex. 5. Discuss the following topics:
1. The Discovery of the Periodic System of the Elements.
2. Mcndelcycv's Life.
3. Mcndclcycv's Interests.
4. The Periodic System of Elements at Present.
DO YOU KNOW THAT...
Mcndclcycv planned to present his paper on the periodic table at the meeting of the Russian Chemical Society on March 6, 1869, but on that day he was ill. His friend N. S. Mcnshutkin (1842-1907), a noted Russian analytical chemist who was a colleague at the University of St. Petersburg read die paper for him. The communication did not evoke any unusual interest at this meeting.
Lesson 3
ГРАММАТИКА: Простое сказуемое в страдательном залоге.
Section I
Ex. 1. Pronounce the following words:
| ЬУ | [ai] | like, nine, side, sky, time, try, by, size, life, final, revise, strike, die, five, type |
| [0 | in, this, with, six, still, little, nickel, mixture, system, crystal, krypton, consist | |
| i + г (+ согласная) | [з:] | first, sir, bird, circle, dirty, girl, third, thirty |
| i + г + гласная | [аю] | fire, wire, entire |
| i + nd | [ai] | kind, mind, find, behind |
b) thorough [*8лгэ], valence fveilons], column fkotoin], immediately [fmiidjoth], accept [bk'scpt], weight [wcit], whereas [weor'aez], isotope ['aisotoop], neutron ['nju:trDn]
c) argon ['agon], cobalt ['кэиЬэ-.lt], gallium ['gatliom], germanium [d3o'meimom], helium ['hi:ljom], iodine [aiodi:n], krypton ['knpton], neon ['ni:on], nickel ['mkl], potassium [po'tacsjom], protactinium [.prooUek'timom], radium f'reidjom], radon ['radon], scandium [skxndiom], tellurium [te'ljuanom], thorium ['Oomom], xenon fzenon]
What are these elements?
Ex. 2. Read the following words and say what Russian words help to understand their meaning:
finalphysical, special^ valencc, column, general, revise, position, indicate, isotope, pij^re^jmass^t^tpn, neutron, vacant, radioactive, contain, group
/•I »i •
18 i ,ci
Ex. 3. Pay attention to the following way of word-building:
основа слова + чмсе (-епсё) [ons] —> существительное
-апсех substance, significance, importance
-encei valence, existence, difference, occurrence, dependence
основа слова + -mcnt [mant] —> существительное
establishment, experiment, development, statement, argument, measurement
основа слова + -ward(s) (wod(z)] наречие aftcrward(s), forward, eastward(s), homeward(s), downward(s)
Ex. 4. Give the initial forms of the following words and find them in the dictionary:
proposed, resembling, placed, discoveries, successes, pairs, occurring, higher, caused, indicated, changing
Ex. 5. Define what parts of speech the italicized words belong to. Translate the sentences into Russian.
1. Every chemical has its own chemical and physical properties. 2. In the evening he usually works in his study. 3. Chemists study properties of elements and their compounds. 4. There is a round table in the middle of the room. 5. Mcndclcycv proposed his periodic table in 1869.6. There arc many places of interest in St. Petersburg. 7. Mcndclcycv places the elements in the order of increasing atomic weight.
Text 3 A
The History of the Periodic Table
The final and most important step in the development of the periodic table was taken in 1869, when the Russian chemist Dmitry Ivanovich Mendeleyev (1834-1907) made a thorough study of the relation between the atomic weights of the elements and their physical and chemical properties, with special attention to valence. Mcndclcycv proposed a periodic table containing seventeen columns, resembling in a general way the present periodic table without the noble gases. In 1871 Mendeleyev revised this table and placed a number of elements in different positions, corresponding to revised values of their atomic weights.
The "zero" group was added to the periodic table after the discovciy of helium, neon, argon, krypton and xenon by Lord Raylcigh and Sir William Ramsay in 1894 and the following years.
The periodic law was accepted immediately after its proposal by Mendeleyev because of its success in making predictions with its use which were afterward verified by experiment. In 1871 Mendeleyev found that by changing seventeen elements from the positions indicated by the atomic weights which had been accepted for them into new positions, their properties could be better correlated with the properties of the other elements.
Most of the elements occur in the periodic table in the order of increasing atomic weights. There still remain, however, four pairs of elements in the inverted order of atomic weight; argon and potassium (the atomic numbers of argon and potassium arc 18 and 19, respectively, whereas their atomic weights are 39.948 and 39.098), cobalt and nickel, tclluruim and iodine, and protactinium and thorium. The nature of the isotopes of these elements is such that the atomic weight of the naturally occurring mixture of isotopes is greater for the clement of the lower atomic number in cach of these pairs than for the clement of higher atomic number; thus, argon consists almost entirely (99.6%) of the isotope with mass number 40 (18 protons, 22 neutrons), whereas potassium consists largely (93.4%) of the isotope with mass number 39 (19 protons, 20 neutrons). This inversion of the order in the periodic system, as indicated by the chemical properties of the elements, from that of atomic weight caused much concern before the atomic numbers of the elements were discovered, but has now been recognized as having little significance.
A very striking application of the periodic law was made by Mcndclcycv. He predicted the existence of six elements which had not yet been discovered, corresponding to vacant places in his table. Three of these elements were soon discovered (they were named scandium, gallium, and germanium by their discoverers), and it was found that their properties and the properties of their compounds are very close to those predicted by Mendeleyev.
After helium and argon had been discovered, the existence of neon, krypton, xenon, and radon was clearly indicated by die periodic law, and the search for those elements in air led to the discovery of the first three of them; radon was then discovered during the investigation of the properties of radium and other radioactive substances.
Words and Word-Combinations to Be Memorized
accept, add, air, application, because of, causc, close, column, compound, conccm, contain, discovery, entirely, following, gas, however, immediately, important, increase, inverse, investigation, isotope, largely, lead, mixture, neutron, noble, occur, pair, physical, propose, proton, recognize, relation, resemble, respectively, revise, search, significance, soon, step, still, striking, success, thorough, vacant, valence, value, weight, whereas
Ex. 6. Give the Russian equivalents for the following:
take steps, make a thorough study, propose, column, in a general way, corresponding to, the following years, acccpt a law, immediately, because of, make predictions, most of, occur, in the order of, still, in the inverted order, respectively, whereas, almost entirely, largely, causc concern, recognize, little significance, application, soon, be close to smth., indicate clearly, during the investigation, radioactive substances
Ex. 7. Give (he English equivalents for the following:
наиболее важный шаг, периодическая таблица, отношение, атомный вес, между, особое внимание, содержать, инертный газ, пересмотреть таблицу, ряд элементов, нулевая группа, добавить к чему-л., успех, большинство элементов, в обратном порядке, атомный номер, смесь изотопов, состоять из, протон, нейтрон, главным образом, большое значение, применение, свободные места в таблице, таким образом, вскоре, химическое соединение, в воздухе, привести к
Ex. 8. Fill in the blanks with prepositions where ncccssary.
by, of, to, in, after, without, between
1. D. I. Mcndclcycv made ... a thorough study ... the relation ... the atomic weights... the elements and their properties. 2. Mcndeleycv's periodic table consisted ... seventeen columns. 3. ... a general way Mcndeleycv's table resembled ... the present periodic tabic ... the noble gases. 4. Mendeleyev placed... a number... elements... different positions. 5. The periodic law was accepted ... its proposal... Mendeleyev. 6. Most... the elements occur ... the periodic table ... the order ... increasing atomic weights. 7. Mcndclcycv predicted the existence ... six elements corresponding... vacant places... his table. 8. The properties... the newly discovered elements were very close ... the properties predicted ... him.
Ex. 9. Translate the sentences into Russian. Pay attention to the meaning of the word that (those).
1. Put your bag on that table. 2. Mendeleyev found that the atomic weights were correlated with the properties of corresponding elements.
3. The atomic weight of potassium is 39.098 and that of argon is 39.948.
4. The inversion of the order in the periodic system from that of atomic weight causcd much concern. 5. The properties of the elements and those of their compounds arc close to those predicted by Mcndclcycv. 6. That difficulty exists no more. 7. The properties of those compounds arc different. 8. Elements from Group I differ from those of Group II.
Ex. 10. a) Write the three forms of the following verbs:
find, have, do, be, mean, know, study, show, bear, lay, draw, found, try, come, take, make, lead, predict, resemble
b) Translate the sentences into Russian.
1. Special attention was drawn to valence. 2. The most important step was taken when Mendeleyev studied the relation between the atomic weights and the properties of the elements. 3. This final step was taken by the great Russian chemist in 1869.4. A periodic table containing seventeen columns was proposed by him. 5. In 1871 the table was revised corresponding to revised values of the atomic weights of some elements.
6. The periodic law was accepted and widely used by chemists. 7. It was found that the atomic weights which had been accepted for some elements were not accurate. 8. After the paper on the periodic table was presented, it was soon published in Russian and in German. 9. Properties of chemical elements and compounds arc thoroughly studied in laboratories. 10. Some elements were given new places in the table after the revision of their' atomic weights. 11. The inversion of die order in the periodic system has now been recognized as having little significance. 12. The periodic law is widely applied by chemists. 13. Radon was discovered during the investigation of the properties of radium and radioactive substances.
Ex. IK Translate the sentences into English.
1. Коща Менделеев разрабатывал периодическую систему, многое элементы еще не были открыты. 2. Русское химическое общество было основано в 1868 году. 3. Нулевая группа была добавлена к периодической таблице после открытия инертных газов. 4. Предсказания Менделеева оправдались последующим открытием новых элементов. 5. Гелий, неон, аргон, криптон и ксенон были открыты в 1894 году и в последующие годы. 6. Всю свою жизнь Менделеев посвятил развитию науки. 7. Химические свойства указывали на нарушение порядка в нескольких местах в таблице. 8. Менделеев предсказал существование шести элементов.
Ex. 12. Make up questions to the italicized parts of the sentences.
1. Mendeleyev proposed a periodic table containing seventeen columns (2). 2. The periodic table was accepted immediately after its proposal (2). 3. Most of the elements occur in the periodic table in the order of increasing atomic weight (2).
Ex. 13. Answer the following questions:
1. When did Mcndclcycv present his periodic system? 2. Were there noble gases in his periodic table? 3. Why did Mendeleyev revise his table? 4. What elements are there in Group "0"? 5. How arc elements arranged in the system? 6. Why arc there elements in the inverted order of atomic weights? 7. What discoveries verified Mcndclcycv's predictions?
Section II
Упр. 1. Прочтите заглавие текста 3 В, бегло просмотрите его и кратко расскажите, о чем он.
Упр. 2. Назовите значения следующих интернациональных слов:
modem, form, systematic, inert, cube, formula, composition, horizontal, period, vertical, group
Упр. 3. Определите значения выделенных слов по контексту.
1. The atomic numbers of helium and neon are 2 and 10, those of lithium and sodium arc one greater (3 and 11). 2. The elements 3,11, 19, 37, 55 and 87 arc very reactive chemically. 3. The sky in summer is often blue, but air is colourless. 4. The properties of the elements Li, Na, K, Rb, Cs and Fr resemble each other, and those of their compounds are similar. 5. The horizontal rows of the periodic table are called periods. 6. The seventh period on which the elements 106 and 107 have a hypothetical existence is called incomplete. 1. Chemists speak about the recurrence of properties, because similar properties do occur again and again in every next period.
Слова к тексту:
cleavage — расщепление; congener — принадлежащий к одному роду; arbitrary — произвольный, случайный; vary — изменяться; dependence — зависимость; connection — связь
Text 3 В
Прочтите текст про себя (контрольное время чтения — 6 минут).
The Periodic Table of the Elements
One of the most valuable parts of chemical theory is the periodic law. In its modern form this law states simply that the properties of the chemical elements are not arbitrary, but depend upon the electronic structure of the atom and vary with the atomic number in a systematic way. The important point is that this dependence involves periodicity that shows itself in the periodic recurrence of characteristic properties.
For example, the elements with atomic numbers 2,10,18,36,54, and 86 are all chemically inert gases. Similarly, the elements with atomic numbers one greater—namely 3,11,19,37,55, and 87 arc all light metals that arc very reactive chemically. These six metals — lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55) and francium (87) — all react with chlorine and form colourless salts that ciystallizc in cubes and show a cubic cleavage. The chemical formulas of these salts arc similar: LiCl, NaCl, KC1, RbCI, CsCl, and FrCl. The composition and properties of other compounds of these six metals arc correspondingly similar, and different from those of other elements.
The horizontal rows of the periodic table arc callcd periods: they consist of a very short period (containing hydrogen and helium, atomic numbers 1 and 2), two short periods of 8 elements each, two long periods of 18 elements each, a very long period of 32 elements, and an incomplete period.
The vertical columns of the periodic table, with connections between the short and long periods as shown, arc the groups of chemical elements. Elements in the same group are sometimes callcd congeners; these elements have closely related physical and chemical properties.
Упр. 4. Скажите, узнали ли вы что-нибудь новое из этого текста. Если да, то что именно?
Упр. 5. Разделите текст на тематические части и озаглавьте их.
Упр. б. Скажите, что говорится в тексте:
1) о строении периодической таблицы; 2) о периодах; 3) о группах элементов; 4) о том, как выявляется периодичность химических и физических свойств элементов.
Унр. 7. Найдите в тексте современную формулировку периодического закона и переведите се на русский язык.
Section III
Ex. 1. Think of the situations when the following expressions may be used:
it's a pity; what a pity; not at all; never mind; I'm not surprised; I don't think so; I have no idea; excuse me (for)
Ex. 2. Translate the sentences into English using expressions from exercise 1.
1. Простите, что я пришел так поздно. — Ничего, я еще долго буду здесь. 2. Петров получил двойку за контрольную работу. — Не могу этому поверить. Он всегда хорошо занимался. 3. Как жаль, что я не приду завтра на ссминар, он будет интересным. — Я так не думаю. 4. Спасибо, что сказали мне об этом. — Не за что. 5. Я читал этот текст с большим интересом. — Не удивительно, он ведь о Менделееве. 6. Завтра в 3 часа будет сообщение о новых результатах исследований. — Жаль, я не приду, у меня в 3 часа лекция. 7. Ты не знаешь, когда он представит свою работу? — Понятия не имею.
Ex. 3. Make up sentences or short stories with the following words:
1) relation, study, weight, property, lead to, periodic, discovciy, table; 2) occur, clement, order, table, periodic, systematic; 3) consist, isotope, aigon, entirely, almost, mass, 40, number, 4) predict, yet, Mcndclcycv, discover, existence, which, element, not, six; 5) periodic, horizontal, table, row, consist, call, period, element; 6) vertical, periodic, column, tabic, group, call
Ex. 4. Give detailed answers to the following questions:
1. What does the periodic law state? 2. How does the periodicity of elements show itself? 3. What do you know about periods of elements? 4. What do you know about groups of elements? 5. Why was the periodic law accepted immediately after its proposal?
Ex. 5. Discuss the following topics:
1. The History of the Periodic System.
2. The Description of the Periodic Table.
3. Predictions Come True.
4. Characteristic Features of One of the Groups of Elements.
DO YOU KNOW THAT...
At present 109 elements are known to the world science, some of them have only hypothetical existence. Attempts arc being made to synthesize them in laboratory. Two elements bear the names of great Russian scientists Mendeleyev and Kurchatov. They arc No. 101 (mcndclcvium) and No. 104 (kurchatovium). The development of the science of periodicity was greatly influenced by the synthesis of transuraniums. Ruthenium (No. 44) was named in honour of Russia.
Lesson 4
ГРАММАТИКА: Особые случаи выражения сказуемого глаголом в страдательном залоге.
Section I
Ex. 1. Pronounce the following words:
|
b) oxygen ['oksid5(a)n], amateur ['acmoto:], adherent [od'hioront], various ['veonos], particularly [po'tikjololi], evidence [ cvidons], phlogiston [flo'dsiston], combustion [kom'bAStJon], concept ['konsopt], average ['acvondsj, atmosphere ['a:tmosfto]
Ex. 2. Read the following words and say what Russian words help to understand their meaning:
religious, pharmacist, component, atmosphere, publication, role, phlogiston, basis, concept, combination, Swedish, French, metal, product
Ex. 3. Pay attention to the following way of word-building:
основа слова + -age [ids] существительное average, heritage, language
основа слова + -iy [ri] —» существительное
theory, ccntuiy, country, factoiy, history, industry, discovciy
основа слова + -П/Жг [tju(:)d] существительное multitude, magnitude
Ex. 4. Arrange the words in the alphabetic order and And their meaning in the dictionary.
credit, share, clergyman, amateur, persecution, derive, delay, ardent, adherent, yield, volume, extent, independently, mercuric
Ex. 5. Define what parts of speech the italicized words belong to. Translate the sentences into Russian. Use the dictionary if necessary.
1. Priestley's work was published in 1774. 2. The students of our group work hard at their English. 3. Sheele's experiments had probably been performed even earlier than Priestley's. 4. He experiments on this substance to make its composition clear. 5. Oxygen plays an important role in combustion. 6. The plays by A. P. Chekhov are known all over the world. 7. Air the room, please. 8. Oxygen makes up 21 per cent of air. 9. The yield of this reaction is usually good. 10. As a result of combustion many substances yield products which react with w ater and give acidic solutions. 11. She looks fine today. 12. Priestley's fine work gave good results.
Text 4 A
Oxygen: History and Occurrence
Credit for the discovery of oxygen is shared by two men, Joseph Priestley, an English clergyman and amateur scientist, who later moved to the United States to escape religious persecution, and Carl Wilhelm Scheele, a Swedish pharmacist. Working independently, these two men both obtained the gas which we know as oxygen by heating various compounds of the clement, particularly mercuric oxide. They also found evidence that this gas is a component of the atmosphere. Priestley's work was published in 1774, but although Schccle's experiments had probably been performed even earlier, their publication was delayed and no account of them appeared until 1777. Though Priestley recognized that the gas which he had discovered plays an important role in combustion, he remained, along with Schccle, an ardent adherent of the phlogiston theory of combustion; in fact, he called the gas "dcphlogisticated air".
On the basis of the experimental results of Priestley, Scheele, and others, as well as some very fine experimental work of his own, in 1777 the brilliant French chemist Lavoisier established the modern concept that the combustion of a substance consists in its combination with the new gas which Priestley and Scheele had described, and which Lavoisier found an important constituent of the atmosphere. Since the combustion of many substances (now known as non-metals) such as phosphorus and sulphur yields products which react with water and give acidic solutions, Lavoisier named this gas oxygen, derived from Greek words meaning "acid former".
Oxygen occurs in the free state as the second most abundant component of the atmosphere; about one-fifth of the air by volume is oxygen. In the combined state it makes up 88.81% by weight of pure water, and, on the average, 85.79% of sea water. It occurs in the earth's crust, in the form of a multitude of compounds, to the estimated extent of46.43%.
Words and Word-Combinations to Be Memorized
abundant, account, acid, air, although, appear, atmosphere, on the average, combination, combustion, component, concept, consist in, constituent, earth, escape, establish, estimate, even, evidence, fine, free, heat, make up, metal, obtain, occurrence, oxide, oxygen, own, particularly, perform, play a role, probably, product, publication, publish, pure, react, since, solution, though, until, very, as well as, yield
Ex. 6. Give the Russian equivalents for the following:
move to, escape smth., obtain, by heating, particularly, find evidence, be a component of smth., although, perform an experiment, even earlier, appear, until, though, along with, in fact, on the basis of, as well as, very fine experimental work, modern concept, work of one's own, consist in smth., since, such as, yield products, an acidic solution, the second most abundant component, about one-fifth by volume, in the combined state, on the average
Ex. 7. Give the English equivalents for the following:
различные соединения, путем нагревания, особенно, найти доказательство, составная часть атмосферы, хотя, опубликовать работу, признавать, играть важную роль, наряду с, на основании, так же как, тонкая работа, современное понятие, заключаться в, так как, неметалл, давать продукты, реагировать с, в свободном состоянии, встречаться в атмосфере, около 90% по весу, чистая вода, в среднем, морская вола
Ex. 8. Fill in the blanks with prepositions and conjunctions where ncccssary.
of у by /л, about, as well as, andb on
1. Scheclc ... Priestley obtained ... oxygen ... heating various compounds ... the clement. 2. Priestley recognized that the gas obtained ... him plays an important role ... combustion. 3. Priestley ... Scheclc
remained adherent... the phlogiston theory... combustion. 4 the basis
... the experimental results ... the earlier workers Lavoisier established his own concept... combustion. 5. The combustion... a substance consists ... its combination with oxygen. 6. Oxygen occurs ... the free state ... the atmosphere. 7___________ one-fifth ... the air ... volume is oxygen.
Ex. 9. Practise the fractional numerals.
a) Простые дроби.
Models 7: — a (one) half
xh — a (one) third
2/э — two thirds
17: — one and a half
2 2/з — two and two thirds
74, Уь, 17«, 107г, 76/$, 127з, 15V4,375,67iz, 47ю
b) Десятичные дроби.
Model: 0.1 — nought point one
0.01 — nought point nought one (point nought one) 2.54 — two point five four
0.2,0.15,1.25,0.001,3.42, 52.03,0.14,0.7
c) Проценты.
Model: 3% — three per ccnt (Latin —pro centum) 2/з% — two fifths per ccnt 0.1 % — nought point one per cent
1%, 7%, 25%, 0.2%, 75%, 27.3%, 81.357%, 16.5%
Ex. 10. Translate into Russian.
a) will be discussed, was being studied, will have been taken, arc named, had been discovered, will be obtained, have been written, were being translated, shall have done, arc used, is being done, will be produced, was asked, are taught, will be given
b) 1. Oxygen was obtained by Scheclc and Priestley independently. 2. Oxygen was obtained by heating mercuric oxide. 3. Evidence was found that this gas is a component of the atmosphere. 4. The theory of phlogiston is not much spoken about at present. 5. An important role of oxygen in combustion was discovered by Priestley. 6. The gas obtained was called by him "dephlogisticatcd air". 7. The modern concept of
combustion was established by Lavoisier. 8. Substances such as phosphorus and sulphur are known as non-metals. 9. The name "oxygen" was derived from Greek words meaning "acid former". 10. Oxygen is found in the free state in the atmosphere. 11. When a substance is attacked by oxygen, it forms an oxide or a number of oxides. 12. Not unfrcqucntly the elements helium, neon, argon, krypton and xenon are referred to as noble gases. 13. Gold is unaffected by oxygen. 14. Many kinds of oxides are met with in the study of chemistry. 15. By Dalton's atomic theory atoms were looked at as indivisible constituents of substances. 16. Potassium is quickly acted on by the oxygen of the air. 17. Oxygen is constantly being put back to the atmosphere by trees and other plants. 18. This method has been followed since the time of Priestley. 19. The lecture was followed by the demonstration of experiments. 20. The first success was followed by many others. 21. The yield of the reaction is greatly affected by temperature. 22. Attention was drawn to the valence of substances. 23. Attempts were made to obtain pure oxygen. 24. Much attention has been given recently to the study of this group of oxides.
Ex. 11. Translate the sentences into English.
1. Современное понятие горения было установлено Лавуазье в 1777 году. 2. Этот газ был назван «образующим кислоты». 3. Кислород был найден в земной коре в виде соединений. 4. Кислород был получен нагреванием различных соединений этого элемента, в частности, оксида ртути. 5. Работа Пристли была опубликована в 1774 году.
6. Кислород был открыт и описан во второй половине XVIII века.
7. Такие вещества, как калий, известны как очень активные металлы.
8. На золото кислород не действует ни при какой температуре.
Ex. 12. Make up questions to the italicized parts of the sentences.
1. Priestley lived and worked in England and then in the United States (3). 2. Oxygen was obtained by heating various compounds of this element (3). 3. The word "oxygen" derived from Greek means "acidformer" (2).
Ex. 13. Answer the following questions:
1. What famous scientists worked on the discovery of oxygen? 2. Who was the first to obtain this gas? 3. What was the method of obtaining oxygen? 4. What theory of combustion existed in Priestley's time? 5. What did the French chemist Lavoisier establish? 6. What docs the modern concept of combustion state? 7. What docs the word "oxygen" mean? 8. Where docs oxygen occur and in what state?
Scction II
Упр. 1. Прочтите заглавие текста 4 В. Бегло просмотрите его и кратко расскажите, о чем он.
Упр. 2. а) Назовите значения следующих интернациональных слов:
essential, respiration, process, diatomic, litre, atmosphere, crystalline, laboratory, accelerate, distillation, control, condition, cylinder, electrolysis, condense
b) Назовите следующие элементы:
potassium, chlorine, manganese, nitrogen, hydrogen
Упр. 3. Определите значения выделенных слов по контексту.
1. Water is a compound with the formula H20. 2. Oxygen supports combustion. 3. A gas which has no colour and odour is called colourless and odourless. 4. One litre of water at 0°C dissolves 48.9 ml of oxygen gas at 1-atm pressure. 5. The boiling point of water is 100°C. 6. Walcvfreezes at 0°C. 7. Above 0°C water is liquid md below 0°C it is solid. 8. Heating potassium chlorate is followed by the evolution of oxygen. 9. When water is boiling, it is evaporating. 10. Oxygen is stored in steel cylinders. 11. When the reaction proceeds very quickly, we usually say that its rate is high. 12. In the laboratory oxygen is obtained by heating KCIO*, and commercially it is made by the distillation of liquid air. 13. Oxygen is soluble in water very little.
Слова к тексту:
fire — огонь; odour — запах; pale — бледны»!; melt — плавиться; amount — количество; volatile—летучий; tend—стрегшпъся
Text 4 В
Прочтите текст про себя (контрольное время чтения — 5 минут).
Oxygen
Oxygen is one of the most abundant elements. It forms 21 per cent of the atmosphere, 89 per ccnt of the water, and about 50 per ccnt of the earth's crust. Without oxygen, life cannot exist, as well as fire. Oxygen is essential in supporting respiration and combustion, it is used in many modern industrial processes. The element consists of diatomic molecules.
It is a colourless, odourless gas, which is slightly soluble in water: 1 litre of water at 0° dissolves 48.9 ml of oxygen gas at 1 -atm pressure. Its density at 0°C and 1 atm is 1.429 g litre'1. Oxygen condenses to a pale blue liquid at its boiling point, -183.0°C, and on further cooling freezes at -218.4°C to a pale blue crystalline solid.
Oxygen is easily prepared in the laboratory by heating potassium chlorate,
KCIO,: 2KC10, -> 2KC1 + 302 (g).
The reaction proceeds at a temperature just above the melting point of potassium chlorate if a small amount of manganese dioxide, MnO:, is mixed with it. Although the manganese dioxide accelerates the rate of evolution of oxygen from the potassium chlorate, it itself is not changed.
Oxygen is made commercially mainly by die distillation of liquid air. Nitrogen is more volatile than oxygen, and tends to evaporate first from liquid air. Nearly pure oxygen is obtained by properly controlling the conditions of the evaporation. Oxygen is stored and shipped in steel cylinders, at pressures of 100 atm or more. Oxygen is also made commercially, together with hydrogen, by the electrolysis of water.
Упр. 4. Скажите, чем отличается текст 4 В от текста 4 А.
Упр. 5. Разделите текст на тематические части и озаглавьте каждую часть.
Упр. 6. Скажите, что говорится в тексте:
1) о распространении кислорода; 2) о его физических свойствах; 3) о получении кислорода в лаборатории; 4) о промышленном получении кислорода; 5) о его хранении.
Упр. 7. Найдите в тексте и переведите на русский язык все предложения, в которых говорится о получении кислорода в промышленности.
Section Ш
Ex. 1. Think of situations when the following expressions may be used:
sorry* I'm sorry, excuse me, that's all right, never mind, not at all, thank you, thanks
Ex. 2. Translate the following sentences into English. Let your fellow students respond to them. Use expressions from exercise 1.
1. Извините, я опоздал. 2. Прости, что я не закончил перевод, меня зовут в лабораторию. 3. Спасибо, что ты помог мне. 4. Извини, я еще не знаю, что буду делать завтра. 5. Спасибо за книгу. Я прочел се за 2 дня. Она очень интересная, б. Жаль, но по химии я получил только «3». 7. Тебе нравится математика? 8.0 чем говорил преподаватель на прошлом занятии?
Ex. 3. Make up sentences or short stories with the following words:
1) be, Priestley, scientist, amateur, English; 2) pharmacist, work, Swedish, Scheele, independently, obtain, gas, oxygen, know, we; 3) they, gas, role, combustion, find, play, evidence, important; 4) French, Lavoisier, modern, combustion, chemist, establish, theory; 5) free oxygen, second, state, occur, most, component, atmosphere, abundant; 6) generally, obtain, liquid, oxygen, commercially, air, distillation; 7) way, oxygen, be, another, obtain, water, electrolysis
Ex. 4. Give detailed answers to the following questions:
1. In what way did Priestley and Scheele obtain oxygen? 2. What is the modern concept of combustion? 3. What is the role of oxygen in combustion? 4. Who established this conccpt first? 5. Is oxygen an abundant element? Give some examples. 6. What are the physical properties of oxygen? 7. What ways of preparing oxygen in the laboratory do you know? 8. How is oxygen made commercially?
Ex. 5. Discuss the following topics:
1. Physical Properties of Oxygen.
2. Chemical Properties of Oxygen.
3. Preparation of Oxygen.
4. The History of the Discovery of Oxygen.
DO YOU KNOW THAT...
The phlogiston theory of combustion was still prevalent in Russia in the middle of the eighteenth century. It came into conflict with the corpuscular theory put forward by Lomonosov. His experiments on the calcination of metals helped Lomonosov to find evidence against it and led him to the discovery of the main law of chemistry — the law of conservation of mass and energy.
Lesson 5
ГРАММАТИКА: Согласование времен.
Scction I
Ex. 1. Pronounce the following words:
|
b) calcium ['katlsiom], carbidc ['kaibaid], commcrcial [кэ'тз:/о1], liquefaction [.hkwi'faskjonj, acctylcnc [o'setili:n], broad [bro:d], field [fi'.ld], garage ['garraij], automobile ['o:tomo,bi:l], manufacture [,macnju'fa:ktJo], refrigerator [ri'fndsorcito], iron ['aion], disease [di'zi:z], alitutudc ['a-ltitjuid), health [hclO], pneumonia [nju(:)'moonjo]
Ex. 2. Read the following words and say what Russian words help to understand their meaning.
interest, gas, active, actual, extremely, factor, speed, method, economically, calcium, carbide, scalc, process, fractional distillation, technically, temperature, revolutionize, defect, operate, industrially, rectification, acetylene, proportion, basis, practically, division, production, garage, automobile, manufacture, refrigerator
Ex. 3. Pay attention to the following way of word-building:
основа слова + -tore [t/э] -> существительное
nature, structure, mixture, feature, picture, temperature, manufacture
but: -sure pressure [-/a], measure [-50]
основа слова (иногда с чередованием гласных корня) + -r/i -> существительное
strength, length, depth, death, breath, health
основа слова + -less —> прилагательное
useless, senseless, colourless, odourless, tasteless, featureless
Ex. 4. Give the initial forms of the following words, arrange them in the alphabetic order and find their meaning in a dictionary.
mixed, welding, divided, fields, perhaps, equipment, applied, surfaces, altitudes, otherwise, feet, besides, required
Ex. 5. Using the context and a dictionary give the right meaning of the italicized words.
1. He usually finds his own way to do the work. 2. There is no other way out. 3. In this way, the oxy-acetylene process is applied almost in every field of industry. 4. How long is a foot? — It is 30.48 centimetres. 5. The phlogiston theory no longer exists. 6. The academic year is divided into two terms. 7. Technical terms are easy to learn if there are Russian analogues. 8. Elements are classified in terms of their properties. 9. My friend lives not far from the University. 10. By far the greatest volume of oxygen is used for industrial purposes.
2 Степаном
Text 5 A
Modern Uses of Oxygen
Although oxygen has been used in industry for more than 100 years, there has been interest in this colourless, odourless, tasteless gas for several hundred years. Its presence as an active element in the air was suspected as long ago as 1500 A. D., but only in 1777 Antoinc Lavoisier, a French chemist, named oxygen and described its properties.
The actual development of the industrial application of oxygen for the next hundred years was extremely slow. Then, at the turn of the twentieth century, two factors greatly speeded progress. One was a method for economically producing oxygen of high purity from the air, and the other was a method for producing calcium carbide on a commercial scalc.
Probably 95 per cent of the huge volume of oxygen used today is obtained from the air by a process which was developed by Dr. Carl von Lindc in Germany in 1895 and 1902. This method is based upon the liquefaction of air and its fractional distillation. Technically, the process is complicated, as it requires one of the lowest temperatures used industrially — more than 300°F below zero (-194.4°C). The liquid air is a very cold mixture of liquid oxygen and liquid nitrogen. Oxygen is then separated by rectification. Most oxygen produced for industrial puiposcs is purer than 99 per cent.
Calcium carbide treated with water produces acetylene, a gas which burns in air with a brilliant white light. When the two gases, oxygen and acctylcnc are mixed in proper proportions and burned, their combustion produces the hottest flame known — more than 5400°F («[2] 3000°C). This flame for the past 100 years has formed the basis for the oxy-acetylcnc process for welding and cutting metals.
Today there is practically no industry which does not use the process. Broadly speaking, applications arc divided into two fields — repair and production. The repair field is perhaps the better known, for practically every garage uses the oxy-acetylcnc process for repairing automobile parts. In industry, there is not a factory which does not use the process in many different ways for repairing the equipment.
Another process where acctylenc is used, is callcd "hard-facing"*. Extremely hard alloys arc applied to the surfaces of metal parts, increasing the life of the parts many times.
Certain industries have developed mostly due to welding. This is true in the manufacture of airplanes, automobiles, refrigerators, railway roads.
But the greatest amount of oxygen is used in cutting iron and steel — one of the most spectacular applications of oxygen in industry. This simple process has literally revolutionized the mctalworking industries. It was found that any cuts were made quite easily.
One of the most recent applications of the oxy-acctylcnc process is for removing surface defects from steel. In this way, larger amounts of cleaner and better steel arc made possible at lower cost.
Although by far the greatest volume of oxygen—amounting to several billion cubic feet a year—is used for industrial puiposcs, an ever increasing amount of oxygen is being used in medicine, the treatment of diseases, such as pneumonia or heart diseases. It has saved many lives. Besides, while breathing oxygen, aircraft pilots operate at altitudes otherwise impractical without it.
It may be said that oxygen is men's best friend — both in industry and for human health.
Words and Vord*Combinations to Be Memorized
acetylene, actual, amount, apply, base, below, besides, bum, calcium, carbide, commercial, complicated, development, distillation, divide, equipment, ever, extremely, factor, foot (feet), fractional, human, interest, iron, liquid, manufacture, mix, nitrogen, operate, past, possible, practically, presence, process, production, proportion, recent, scale, separate, speed, steel, taste, tasteless, technically, temperature, than, true, at the turn of the century, while
Ex. 6. Give the Russian equivalents for the following: long ago, more than, less than, industrial application, extremely slow, at the turn of the twentieth century, speed progress, high purity, on a commercial scale, huge volume, obtain by a process, develop a process, liquid air, separate, for industrial purposes, treat with smth., mix in proper proportions, form the basis for smth., automobile parts, the mctalworking industries, remove a defect, treat a disease, breathe oxygen
Ex. 7. Give the English equivalents for the following: активный элемент, только, в промышленных масштабах, вероятно, карбид кальция, получить из воздуха, разработать метод, фракционная перегонка, самая низкая температура, ниже нуля, выше нуля, жидкий воздух, для промышленных целей, гореть на воздухе, разделить на, металлические детали, наибольшее количество, простой процесс, таким образом, возможный, хотя, кроме того, лучший друг человека
Ex. 8. Fill in the blanks with prepositions where necessary. of у at, in, for, from, upon, with
1. Oxygen has been used ... undustry ... more than 100 years. 2. The actual development... the industrial application ... oxygen began ... the turn of the twentieth century. 3. Today probably 95 per ccnt... the huge volume ... oxygen is obtained ... the air. 4. This method is based ... the liquefaction ... air and its fractional distillation. 5. When calcium carbide is treated ... water, it produces acetylene. 6. Acetylene burns ... air ... a brilliant white light. 7. The greatest amount... oxygen is used ... cutting iron and steel.
Ex. 9. a) Cheek up if you remember the following:
|
| b) Translate the following adjectives into English and give their comparative and superlative forms: |
новый, хороший, большой, плохой, интересный, теоретический, много, легкий, высокий, маленький
c) Translate the sentences into Russian, paying attention to the adjectives.
1. The Russian language is more difficult than the English language. 2. This work is less important than that work. 3. Lesson 2 is more interesting than Lesson 1.4. What is the largest city in Russia? 5. Is the Volga longer than the Lena? 6. Which is the oldest building in St. Pctcrsbuig University?
7. Which was the most difficult subjcct for you when you were at school?
8. Who is the oldest in your group? 9. Most oxygen produced for industrial purposes is purer than 99 per cent. 10. Some applications of oxy-acetylcnc process arc better known than others. 11. One of the most spectacular applications of oxygen in industry is for cutting iron and steel. 12. Perhaps you will describe the most recent applications of the oxy-acetylcne process. 13. Oxygen is men's best friend.
Ex. 10. Translate the sentences into Russian.
1. He told me that the lecture would begin at 3 p. m. 2. They knew student N was working at the laboratory. 3. As I hadn't read this article before, I went to the reading-room. 4. I thought she would become a good student. 5. When I came to the laboratory, he had already gone home. 6. We were told that even Lavoisier had been interested in oxygen. 7. The teacher explained how the substance had been obtained in his laboratory. 8. The hypothesis was very interesting but we wanted to know how it had been developed. 9. Soddy showed that some radioactive elements had similar behaviour. 10. Mcndclcycv prcdictcd that more elements would be discovered and even described their properties. 11. Early chemists thought that water was an clement. 12. Their experiment showed that not all isotopes were stable. 13. When we came in, she had finished her experiment and was analysing the results. 14. Everybody knew that professor N was making experiments on the properties of substances.
Ex. 11. Translate the sentences into English.
1. Кислород уже давно применяется в промышленности. 2. Кислород обычно получают из воздуха путем его сжижения и фракционной перегонки. 3. В техническом отношении этот процесс сложен. 4. Жидкий воздух — это очень холодная смесь жидкого кислорода и жидкого азота. 5. Когда карбид кальция обрабатывают водой, получается ацетилен, б. В настоящее время кислородно-ацетиленовая сварка и резка металлов применяется почти во всех отраслях промышленности. 7. Огромное количество кислорода используется ежегодно для промышленных целей. 8. Кислород—лучший друг человека. Он послужил спасению жизни многих людей.
Ex. 12. Make up questions to the italicized parts of the sentences.
1. There has been interest in oxygen for several hundred years (2). 2. Today there is practically no industry which does not use the oxy- acetylene process (3). 3. Applications of the oxy-acetylene process arc divided into two fields — repair and production (3).
Ex. 13. Answer the following questions:
1. Who gave the name to oxygen? When? 2. What can you say about the industrial application of oxygen in the nineteenth century? 3. What industrial application of oxygen is known best of all? 4. In what fields of industry is the oxy-acctylenc process used? 5. Why is oxygen called men's best friend?
Scction II
Упр. 1. Прочтите заголовок и подзаголовок текста 5 В. Вспомните к скажите, что вы уже знаете по данной теме.
Упр. 2. Назовите значения следующих интернациональных слов:
radiation, ultraviolet, ozone, clectric, arc, generation, electrolysis, concentration, efficiency, characteristic, detect, toxicity, molecule, linear, contract, diamagnctic, resonance, clectrophilic, mechanistic, biological, effect, vitamin, observation, antioxidant, protection, destructive, elastomer
Упр. 3. Определите значения выделенных слов по контексту. Синонимы в скобках помогут вам.
1. Electrolysis is one of the common (usual) methods for obtaining ozone. 2. The ultraviolet radiation technique (method) is used when relatively (comparatively) low concentrations arc obtained. 3. When high- purity ozone is desired (is ncccssary), liquid ozone is used. 4. When water is boiling, vapour is formed. 6. The normal atmospheric pressure is 760 mm. 7. The characteristic odour of ozone is detectable by nose. 8. This odour provides (gives) a warning (a signal) of toxicity. 9. On exposure to oxygen metals arc oxidized. 10. Gaseous ozone possesses four resonance structures. 11. Elcctrophilic nature of ozone is accounted for (explained by) this fact.
Слова к тексту:
silent discharge — тихий разряд; require — требовать; environment — окружающая среда; pass through a vessel — проходить через сосуд; bond — связь; rat — крыса; undergo — подвергаться
Text 5 В
Прочтите текст про себя (контрольное время чтения — 7 минут).
Ozone: Properties, Toxicity, and Applications
Common methods for ozone generation are ultraviolet radiation, silent electric arc discharge and electrolysis. The unltraviolct radiation technique is used only when relatively low concentrations arc required, c. g. for environmental study. The electrolysis of water is not widely used because of its low cfficicncy. So far, the action of silent electric discharge on oxygen is the most common method for the production of ozone. It gives a yield of 6% by weight of ozone.
When high purity or quantity of ozone is desired, it is possible to use liquid ozone. The ozone-oxygen mixture from one of the above methods is passed through a vessel at liquid oxygen temperature (-183°C). Because of the large difference in the vapour pressure of ozone and oxygen, oxygen is pumped off from the mixture whereas ozone is liquefied. Liquid ozone is then vaporized from the conccntrated system for further use.
Ozone is a colourlcss gas with characteristic odour which is detectable by nose and provides a warning of toxic exposure. The ozone molcculc is non-linear with a bond angle of 116°. In contrast to oxygen, gaseous ozone is diamagnctic and possesses four resonance structures with (III) and (IV) accounting for its clectrophilic nature.
0* 0- 0 0 Л / / /
-о 0 0 0- 4) о- -о 0♦ I II III IV
Mechanistic studies of the biological effect of ozone are of particular interest. Vitamin E prolongs the life of rats exposed to ozone. This is based on the observation that the vitamin E-dcplctcd group undergoes a more significant change than the vitamin E-supplcmcntcd group in the fatty acid composition of total lung lipid. Therefore vitamin E, the biological antioxidant, provides protection from ozone toxicity.
Ozone is a particularly destructive substance to rubber and other elastomers.
Упр. 4. Скажите, узнали ли вы что-нибудь новое из этого текста. Что именно?
Упр. 5. Разделите текст на тематические части и озаглавьте каждую часть.
Упр. б. Скажите, что говорится в тексте:
1) о получении озона; 2) о том, какой метод получения озона является самым распространенным; 3) о том, что собой представляет озон; 4) о том, каково биологическое действие озона.
Упр. 7. Найдите в тексте предложения с описанием строения молекулы озона и переведите их на русский язык.
Section 1П
Ex. 1. a) Think of the situations when the following or similar expressions may be used:
so do I, so shall I, so is he, etc.; neither do I, neither shall I, neither is he, etc.
b) Translate the following dialogues into Russian:
1.1 like reading very much. — So do 1.2. He goes to the cinema once a week. — So docs she. 3. They have already had breakfast. — So have we. 4.1 don't like examinations. — Neither do I. 5. He doesn't go to the theatre very often. — Neither docs his sister. 6. They haven't visited Mcndclcycv's museum yet. — Neither have we.
Ex. 2. Translate the sentences into English. Let your fellow students respond to them. Use expressions from exercise 1.
1. Мы знаем много законов химии и физики. 2. Я не помню значения этого слова. 3. Нам нравится читать текст о великих ученых. 4. Мы узнали много интересного о Менделееве. S. До сих пор я никогда не слышал о Пристли, б. Он еще не знает, что мы уже работаем в лаборатории. 7. Мне не понравилась последняя лекция по математике.
Ex. 3. Make up sentences or short stories with the following words:
1) oxygen, almost, industry, use, every, field; 2) amount, use, cutting, iron, great, oxygen, steel; 3) oxygen, diseases, amount, ever, use, increasing, treatment; 4) oxy-acctylcnc, application, know, process, repair, production, field; 5) gas, characteristic, be, ozone, colourless, odour
Ex. 4. Give detailed answers to the following questions:
1. What factors speeded die development of the industrial application of oxygen? 2. How is oxygen produced today? 3. Why has the oxy-acctylcnc proccss found wide application? 4. Where else is oxygen used besides in industry?
Ex. 5. Discuss the following topics:
1. Methods of Obtaining Oxygen.
2. The Application of Oxygen.
3. The Difference Between Oxygen and Ozone.
DO YOU KNOW THAT...
In nature ozone is formed from oxygen during lightning discharges, and at altitudes of 10,000-30,000 metres through the action of solar ultraviolet radiation. Ozone removes harmful ultraviolet radiation from sunlight and also absorbs the Earth's own infra-red radiation thus protecting it against cooling. Therefore the ozone belt plays a major role in protecting life on Earth.
Lesson 6
ГРАММАТИКА: Составное именное сказуемое. Общие сведешм о неличных формах глагола. Способы выражения предикатива.
Section I
Ex. 1. Pronounce the following words:
|
b) light [lait], ratio ['rcijiou], committee [ko'miti], accuracy [arkjorosi], deuterium [dju(:)'ttonom], tritium ('tritiam], nucleus ['nju:klios]( ionize ['aianatz],
covalent [koo'veilont], convenient [kon'virnjont], sulphuric [SAl'fjoorik], cathode ['kscOaod], anode [snood]
Ex. 2. Read the following words and say what Russian words help to understand their meaning:
mass, individual, calculate, committee, standard, zinc, impress, isotopic, deuterium, tritium, radioactive, stable, ordinary, separation, electrolysis, concentrate, apparatus, proton, ion, sole, neutron, characteristic, general, natural, recombinc, line, combination, organic, covalent, type, class, act, negative, molecule, hydride, barium, action, cathode, anode
Ex. 3. Pay attention to the following way of word-building: основа слова + -ize [aiz] —> глагол
summarize, organize, recognize, ionize, revolutionize, generalize
основа слова + -де/ существительное, прилагательное
student, component, constituent, coefficient, opponent
different, convenient, intelligent, efficient, violent, evident, constituent
со- -i- основа слова существительное, прилагательное» глагол co-worker, co-author, co-pupil, co-student, co-reactant, coordination
covalent, coaxial, coordinate ____________ coexist, cooperate, coagulate, coordinate
Ex. 4. Compare the meanings of the verb with and without prepositions. Use a dictionary if necessary.
come — come back — come off— come out; decide — decide upon; go — go back — go on — go away — go out — go in for; stand — stand up — stand for, make—make up — make out; find — find out; set — set up; give—give away—give back—give off— give up; get—get off— get out — get together — get up; consist in — consist of; use—use up
Ex. 5. Make up some sentences of your own illustrating different meanings of verbs with and without prepositions. Use the verbs from exercise 4.
Text 6 A Hydrogen
Hydrogen is the lightest chemical clement. Its mass is the unit of measurement for the masses of other elements.
Atomic weight, or mass, was long considered the most important property of an clement. By weighing the amounts of individual elements making up a chemical compound and calculating the weights of these ratios
to the weight of hydrogen which will combine with the same elements, it is found that the atomic weights of the other elements are almost, but not quite, whole numbers.
During the nineteenth ccntury a committee of chemists was chosen to decide upon a standard of accuracy for atomic weights. The committee set the atomic weight of oxygen at 16.000 in order to make the atomic weights of other elements come out closer to whole numbers. That change of standards gave hydrogen the weight of 1.008.
Hydrogen was first obtained in 1766 by Sir Henry Cavendish in London. He found that he could get the gas by dissolving zinc, iron or tin in diluted vitriolic acid (H2S04) or spirit of salt (HCI). He discovered that a mixture of hydrogen and common air explodes with a long noise, and he was impressed with the lightness of the gas. He named the gas "inflammable air", the name "hydrogen" (water-former) was given by Lavoisier.
Hydrogen exists in three isotopic forms, known as hydrogen (or protium), deuterium and tritium. Tritium is radioactive, with a short half-life. Deuterium is stable, and occurs in a small amount with ordinary hydrogen. Its compound (D20) is known as heavy water. Slight differences between the properties of ordinary water and heavy water allow their separation, notably by electrolysis, in which ordinary water is decomposed and heavy water bccomcs concentrated in the water left in the apparatus.
The nuclcar structure of ordinary hydrogen consists of one proton, the unit of matter. This is the same as a hydrogen ion. An electron as the sole planet in this system completes the structure of hydrogen atom.
The difference between ordinary hydrogen and heavy hydrogen (deuterium) lies in the fact that deuterium has a neutron in the nucleus in addition to the proton. Addition of the neutron adds weight but docs not changc the chemical characteristics. This is in accordance with a general rule covering structures of elements.
Tritium has a nucleus consisting of one proton and two neutrons.
Hydrogen is given off by some natural gas wells, but it escapes into the upper air. It is not found uncombincd on the earth. It is recognized in the stars by its spectrum lines in the light that we rcccive from them.
In combination with oxygen, in the form of water, and with carbon, in the many organic compounds, hydrogen is one of the most abundant elements on the earth.
Hydrogen combines with other elements and forms different kinds of compounds, some of which ionize in solution, others which arc joined with covalcnt bonds, yielding organic types of compounds.
Although formerly it used to be classcd with the alkali metals of Group I in the periodic table, hydrogen acts as a negative part of the molcculc when it is in combination with those metals. It forms hydrides which arc in general colourlcss crystals. Similar compounds arc formed with calcium and barium of Group II. The hydrides decompose in water,
releasing hydrogen. This property has been used as a convenient way to store hydrogen.
Hydrogen is usually obtained by action of sulphuric acid (H2SO4) on zinc. The metal replaces the hydrogen, which bubbles ofT a gas. Electrolysis of water also liberates hydrogen at the cathode, while oxygen comes off at the anode.
Words and Word-Combinations to Be Memorized
in accordance with, act, action, alkali, anode, apparatus, barium, bond, carbon, choose, comc out, combine, concentrate, consider, covalent, decompose, difference, dissolve, electrolysis, electron, explode, form, general, give off, half- life, hydride, hydrogen, ion, ionize, line, measurement, molecule, negative, nuclcus, in order to, ordinary, radioactive, separation, spectrum, stable, standard, sulphuric, tin, unit, whole, zinc
Ex. 6. Give the Russian equivalents for the following:
unit of measurement, make up a compound, whole numbers, a committee of chemists, a standard of accuracy, in order to, weigh, dissolve, zinc, iron, a diluted acid, common air, the lightness of the gas, hydrogen, an isotopic form, radioactive, a short half-life, stable, a small amount, ordinary, heavy water, slight difference, electrolysis, apparatus, consist of, the unit of matter, an ion, an electron, complete the structure, the difference lies in the fact, in addition to the proton, change the chemical characteristics, be in accordance with, a rule, a neutron, a proton, to give off, escape into the upper air, in the stars, spectrum lines, different kinds of compounds, in solution, similar compounds, at the cathode, at the anode
Ex. 7. Give the English equivalents for the following:
самый легких! химический элемент, единица измерения, самое важное свойство, целое число, для того чтобы, находить, получить газ, растворить, разбавленная серная кислота, обычный воздух, легкий газ, изотопная форма, радиоактивный, короткий период полураспада, химическое соединение, тяжелая вода, незначительные различия, электролиз, состоять из, находиться в соответствии с, общее правило, линии спектра, в виде воды, соединяться с другими элементами, различные соединения, ионизироваться в растворе, щелочные металлы, гидрид, разлагаться в воде, на катоде, на аноде
Ex. 8. Fill in the blanks with prepositions where necessary.
on, by of у in, withy between, to, out, at, in order to > for
1. The mass ... hydrogen is the unit... measurement... the masses ... other elements. 2. The committee set the atomic weight ... oxygen ... 16.000 to make the atomic weights ... other elements comc ... closer ... whole numbers. 3. Hydrogen was given the weight... 1.008.4. Hydrogen was obtained ... 1766 ... Henry Cavendish ... London. 5. Cavendish
obtained ... hydrogen... dissolving... zinc, iron or tin ... diluted H2S04 or HC1.6. Cavendish was impressed ... the lightness ... the gas. 7. The name "hydrogen" was given ... Lavoisier. 8. Hydrogen exists ... three isotopic forms. 9. There arc slight differences ... the properties ... ordinary water and heavy water. 10. Hydrogen is one... the most abundant elements... the earth. 11. Hydrogen combines ... other elements and forms different kinds ... compounds. 12. Some ... the hydrogen compounds ionize ... solution. 13. Hydrogen is usually obtained ... action ... sulphuric acid ... zinc.
Ex. 9. a) Fill in the blanks with some, any, no.
1. There is ... acid in this glass. 2. Have... more tea, will you? 3. Will you give mc ... books now? 4. Sorry, but I have ... pen here. 5. Have ... students come already? 6. They haven't read ... texts yet. 7. Arc there... books on your table? 8. Is there ... diffcrcncc between oxidation and combustion? 9. There is ... gas in this container, it has cscapcd. 10. She has ... work to do in the evening.
b) Put the sentences into interrogative and negative forms, give short answers to the obtained questions.
1. There are some students in the room. 2. There arc some mistakes in his test-paper. 3. There arc some books on the table. 4. There is some information on this process. 5. There is some oxygen in the tube. 6. There arc some interesting articles in this journal.
Ex. 10. Translate the sentences into Russian; say what the predicatives are expressed by.
1. Hydrogen is the lightest chemical element. 2. The mass of hydrogen is the unit of measurement for the masses of other elements. 3. The atomic weights of the other elements are almost whole numbers. 4. The atomic weight of oxygen is 16.000. 5. Tritium is radioactive. 6. The formula of heavy water is D20.7. Hydrogen is one of the most abundant elements on the earth. 8. In general hydrides arc colourless ciystals. 9. The abbreviation of gram is "g". 10. The properties of the elements of Group I arc nearly the same. 11. Chemistry is one of the natural scicnccs. 12. The action of oxygen is to oxidize matter. 13. The purpose of chemical analysis is to determine the composition of compounds. 14. To dilute an acid is to make it less concentrated. 15. Gases arc difficult to weigh. 16. Molcculcs of a gas are free to move in the gas container. 17. On exposure to air activc metals bccomc oxidized. 18. A characteristic property of hydrogen is that it exists in three isotopic forms. 19. The chemical behaviour of an clement is how it acts toward other elements. 20. The question is how much hydrogen we have obtained.
Ex. 11. Translate the sentences into English.
1. Водород был впервые получен в 1766 году. 2. Его получнл Генри Кавсндиш, который в то время работал в Лондоне. 3. Он получал водород растворением цинка, железа или олова в разбавленной серной или соляной кислоте. 4. Комитет решил установить атомный вес водорода 1,008, а кислорода — 16,000. 5. Водород — самый легкий элемент. 6. Название «водород» означает «образующий воду». 7. Ученые знают три изотопных формы водорода. 8. Одна из них (тритий) — радиоактивна, с коротким периодом полураспада. 9. Водород—один из наиболее распространенных элементов на Земле. 10. Гидриды разлагаются в воде, испуская водород.
Ex. 12. Make up questions to the italicized parts of the sentences.
1. Hydrogen is recognized in the stars by its spectrum lines in the light that we receive from them (2). 2. Hydrogen forms different kinds of compounds, some of them ionize in solution (3). 3. Hydrogen forms hydrides which arc in general colourless crystals (2).
Ex. 13. Answer the following questions:
1. What kind of gas is hydrogen? 2. Why was this gas named "water- former'*? 3. Why was hydrogen chosen the unit of measurement for the masses of other elements? 4. What arc the isotopic forms of hydrogen? 5. What is the structure of a hydrogen ion? 6. Docs hydrogen occur free in nature? 7. In what forms docs hydrogen mostly occur in nature? 8. How is hydrogen usually obtained?
Section II
Упр. 1. Прочтите заголовок текста 6 В. Скажите, чем, по вашему мнению, он б>дет отличаться от текста 6 А?
Упр. 2. Назовите значения следующих интернациональных слов:
substance, inorganic, helium, litre, laboratory, reaction, generate, industry, convert, oxide, regenerate, monoxide, special, reason, clcctro- negatively
Упр. 3. Определите значения выделенных слов по контексту. Синонимы в скобках помогут вам.
1. Hydrogen is a very widely distributed (abundant) clement. 2. The density of a substance is mL 3. The solubility of a substance is how readily it dissolves. 4. When the solubility becomes greater, we say that it increases; when it becomes lower, we say that it decreases. 5. The symbols for sodium and lead arc Na and Pb. 6. Alkali metals react very vigorously (intensively) with water.
Слова к текст)':
equation — уравнение; steam — нар; heat — теплота; ignite — воспламенить; filings — металлические опилки, стружка
Text 6 В
Прочтите текст про себя (контрольное время чтения — 5 минут).
Hydrogen Production
Hydrogen is a very widely distributed element. It is found in most of the substanccs that constitute living matter, and in many inorganic substanccs. There arc more compounds of hydrogen known than of any other element.
Free hydrogen, H2, is a colourless, odourless, and tasteless gas. It is the lightest of all gases, its density is about one-fourteenth that of air. Its melting point (-259°C or 14K) and boiling point (-252.7°C) arc very low, only those of helium arc lower. Liquid hydrogen, with density 0.070 gfcnr3, is the lightest of all liquids. Crystalline hydrogen, with density 0.088 g/cm~3, is also the lightest of all crystalline substances. Hydrogen is very slightly soluble in water, 1 litre of water at 0°C dissolves only 21.5 ml of hydrogen gas under 1-atm pressure. The solubility decreases with increasing temperature, and increases with the increase in the pressure of the gas.
In the laboratory, hydrogen is easily made by the reaction of an acid such as sulphuric acid, H2S04, with a metal such as zinc. The equation for the reaction is:
H2S04(aq) + Zn(c) ZnS04(aq) + H2(g).
Sometimes hydrogen is prepared by the reaction of some metals with water or steam. Sodium and the other alkali metals react very vigorously with water, so vigorously as to generate enough heat to ignite the liberated hydrogen. An alloy of lead and sodium, which reacts less vigorously, is sometimes used for the preparation of hydrogen.
Much of the hydrogen that is used in industry is produced by the reaction of iron with steam. The steam from a boiler is passed over iron filings heated to a temperature of about 600°C. The reaction that occurs is
3Fc(c) + 4H20(g) Fc,04(c) + 4H2(g).
After a mass of iron has been used in this way for some time, it is largely converted into iron oxide, Fcj04. The iron can then be regenerated by passing carbon monoxide, CO, over the heated oxide:
Fcj04(c) + 4CO(g) 3Fc(c) + 4C02(g).
There is, of coursc, nothing special about sodium and iron, except their low cost and availability, that is the reason why they arc used for the preparation of hydrogen. Other metals with electronegativity about the same as that of sodium (x=0.9) react with water as vigorously as sodium, and metals with electronegativity about the same as that of iron (x=1.8) react with steam in about the same way as iron.
Упр. 4. Скажите, подтвердились лн ваши предположения о содержании текста? В чем?
Упр. 5. Разделите текст на тематические части и озаглавьте каждую часть.
Упр. 6. Скажите, что говорится в тексте:
а) о распространении водорода; б) о физических свойствах водорода.
Упр. 7. Найдите в тексте описание получения водорода в промышленности и переведите этот отрывок на русский язык.
Section III
Ex. 1. Think of the situations when a disjunctive question may be used Examples:
1)She is the first-year student, isn't she? — Yes, she is. You were absent yesterday, weren't you? — Yes, I was. You have done the exercise, haven't you? — Yes, I have.
2) She hasn't finished her speech, has she? — No, she hasn't. We haven't yet studied such reactions, have we?—No, we haven't
Ex. 2. Translate the following questions into English. Let your fellow- students respond to them.
1. Мы уже много знаем о водороде и кислороде, не правда ли?
2. Химические свойства щелочных металлов очень интересны, да?
3. Вы еще не закончили свою лабораторную работу, да? 4. Он прав, не правда ли? 5. Эта книга не очень трудна для вас, не правда ли? 6. Вам нравится учиться, правда? 7. Она живет на Среднем проспекте, да? 8. Вы уже видели своего преподавателя сегодня, да? 9. В аудитории № 21 нет сейчас студентов, не правда ли? 10. У нас уже не осталось времени, да?
Ex. 3. Make up sentences or short stories with the following words:
1) hydrogen, element, unit, other, be, it, use, lightest, mass chemical, measurement, clement; 2) first, dissolve, iron, Cavendish, obtain, zinc, tin, hydrogen, acid, diluted, sulphuric; 3) be, clement, earth, hydrogen, abundant, spectrum, find, line, stars; 4) prepare, some, metal, at present, hydrogen, reaction, water
Ex. 4. Give detailed answers to the following questions:
1. What arc the physical properties of hydrogen? 2. In what way was hydrogen obtained first? 3. What arc the chemical properties of hydrogen?
4. What do you know about the isotopic forms of hydrogen? 5. What arc the ways of obtaining hydrogen?
Ex. 5. Discuss the following topics: 1. The Structure of a Hydrogen Atom.
2. Hydrogen as the Unit of Measurement for the Masses of Other Elements.
3. The Difference Between the Isotopic Forms of Hydrogen.
4. Commercial Obtaining of Hydrogen.
DO YOU KNOW THAT...
In accordancc with the theory of acids existing at that time, the result of the reaction of inflammable air and oxygen was cxpcctcd in the form of an acid, but what Lavoisier obtained was only water. Later he learnt that Henry Cavendish in London had made similar experiments and had obtained water as a result of a combustion of the mixture of "inflammable air" and oxygen. Lavoisier repeated the experiment and made a striking discovery that water was not an element. And he gave "inflammable air" the name of hydrogen.
Lesson 7
ГРАММАТИКА: Составное именное сказуемое. Типы глаголов-связок.
Section I
Ex. 1. Pronounce the following words:
|
b) silicon ['silikon], dioxide [dai'Dksaid], mineral ['minorol], quartz [kwo:ts], hexagonal [hek'satgonl], identify [ai'dcntifai], polarization [#pootorai'zciJn], silicic [sf hsik], hydroxyl [haid'roksil], adjacent [o'd3eisont], neighbour ['neibo]
Ex. 2. Read the following words and say what Russian words help to understand their meaning.
dioxide, mineral, quartz, granite, rotate, polarization, condensation, product, ordinary, position, cubic
Ex. 3. Pay attention to the following way of word-building:
основа слова + -rtess [ms] -»существительное
lightness, usefulness, hardness, beautifulness, happiness, whiteness
основа слова + -ty [ti] -» существительное
party, property, speciality, purity, beauty
основа слова + -fy [fai] -> глагол
verify, testify, intensify, signify, identify, simplify
Ex. 4. Find the meaning of the following words in a dictionary. If you can't find the word, try to find its constituent parts and deduce the meaning of the whole word.
hexagonal, widespread, well-formed, right-handed, left-handed, tetrahedron, test-tube, cross-road, test-paper, fellow-student, water-proof, shop-assistant
Ex. 5. Find the meaning of the italicized words in a dictionary and translate the sentences into Russian.
1. Quartz occurs in many deposits as well-formed crystals. 2. It also occurs as a crystalline constituent of many rocks. 3. The crystals of quartz are left-handed or right-handed by their face development. 4. Silicic acid has the property of undergoing condensation very radily with elimination of water. 5. Each silicon atom is surrounded by four oxygen atoms. 6. The structure of quartz accounts for the hardness of the mineral.
Text 7 A Silicon Dioxide
Silicon is, next to oxygen, the most abundant clement in the earth's crust which occurs mostly in the form of oxides. Silicon dioxide (silica), Si02, occurs in nature in three different crystal forms: as the minerals quartz (hexagonal), cristobalite (cubic), and tridymitc (hexagonal). Quartz is the most widespread of these minerals; it occurs in many deposits as well-formed crystals, and also as a crystalline constituent of many rocks, such as granite. It is a hard, colourless substance. Its crystals arc identified as right-handed or left-handed by their face development
and also by the direction in which they rotate the plane of polarization of polarized light.
The structure of quartz is closcly related to that of silicic acid, H4Si04. In this acid silicon has ligancy 4, and is surrounded by a tetrahedron or four oxygen atoms, with one hydrogen atom attached to each oxygen atom. Silicic acid, which is a very weak acid, has the property of undergoing condensation very readily, with elimination of water. If each of the four hydroxyl groups of a silicic acid molcculc condenses with a similar hydroxyl group of an adjaccnt molecule, eliminating water, a structure is obtained in which the silicon atom is bonded to four surrounding silicon atoms by silicon-oxygcn-silicon bonds. This proccss leads to a condensation product with formula Si02, sincc each silicon atom is surrounded by four oxygen atoms, and each oxygen atom serves as a neighbour to two silicon atoms. The structure of quartz and of the other forms of silica is described as consisting of Si04 tetrahedra, and cach oxygen atom is a corncr of two of these tetrahedra. In order to break a crystal of quartz it is ncccssary to break some silicon-oxygcn bonds. In this way the structure of quartz accounts for the hardness of the mineral.
Cristobalitc and tridymitc are similarly made from Si04 tetrahedra fused together by sharing oxygen atoms, with, however, different arrangements of the tetrahedra in spacc from that of quartz. Tridymitc resembles ordinary icc in stmcturc, with silicon atoms in the oxygen-atom positions; cristobalitc similarly resembles cubic icc.
Words and Word-Combinations to Be Memorized
account for, adjacent, arrangement, attach, closely, crystalline, deposit, dioxide, formula, fuse, granite, group, hydroxyl, identify, mostly, neighbour, position, quartz, be related to, serve, share, silicon, similarly, since, space, surround, together, undergo, weak
Ex. 6. Give the Russian equivalents for the following:
next to oxygen, mostly, the most widespread mineral, deposit, a well- formed crystal, constituent, a hard substance, identify something, right- handed, left-handed, rotate, the plane of polarization, polarized light, be closely related to, be surrounded by, a weak acid, undergo condensation, a hydroxyl group, an adjaccnt molccule, be bonded to, lead to, condensation product, neighbour, in order to break a crystal, account for, be fused together, share oxygen atoms, ordinary icc, resemble smth. in structure
Ex. 7. Give the English equivalents for the following:
диоксид кремния, встрсчапгься в природе, вращать плоскость поляризации, структура кварца, быть тесно связанным с, окружать, присоединить к атому, очень слабая кислота, подвергаться конденсации, очень легко, каждая из гидроксильных групп, молекула кислоты, связь, вести к, продукт конденсации, разорвать связь, таким образом, объяснять, твердость минерала, делить между собой атомы кислорода, различные расположения в пространстве, быть похожим на лсд по структуре
Ex. 8. Fill in the blanks with prepositions where necessary.
о/$ for, in, with, to j by
1. Silicon occurs mostly ... the form ... oxides. 2. Silicon dioxide occurs... nature... three different crystal forms. 3. The structure of quartz is closely related ... that... silicic acid. 4. Silicic acid has the property ... undergoing condensation ... elimination ... water. 5.... Si02 each silicon atom is surrounded ... four oxygen atoms. 6. To break a crystal... quartz it is ncccssaiy to break... some silicon-oxygen bonds. 7. The structure... quartz accounts ... the hardness ... the mineral.
Ex. 9. Check up if you remember the following:
a) Words of the Greek or Latin origin form their plural by changing the suffixes in the following way:
The following nouns have the same forms of their singular and plural: apparatus, means, news, series, species |
b) Make up singular-plural pairs from the list below:
quanta, maximum, analyses, vacua, axis, maxima, nuclei, analysis, apparatus, criteria, nucleus, quantum, matrix, crisis, theses, crises, moments, axes, syntheses, criterion, thesis, momentum, synthesis, vacuum, matrices, apparatus
c) Translate the sentences into Russian paying attention to the italicized nouns.
1. Until about 1860 the majority of chemists used formulae merely as a convenient representation of organic compounds. 2. We heard a very good news today. 3. The phenomenon of attraction of oppositcs interested many philosophers. 4. Neutrons arc also the most effective means of producing artificial radioactivity. S. Avogadro's hypothesis was originally advanced to explain Gay-Lussac's law of combining volumes. 6. There is some new apparatus on that table. 7. To analyse species is to identify their constituent elements. 8. There are eighteen elements in this series.
Ex. 10. Translate the sentences into Russian paying attention to link-verbs.
1. Quartz is a hard, colourlcss substancc. 2. Silicic acid is a weak acid.
3. To break a crystal of quartz is to break some silicon-oxygcn bonds.
4. Silicon mostly occurs combined. 5. As chcmistry developed, some hypotheses became laws. 6. An acid turns blue litmus red. 7. Water turns into icc at 0°C. 8. Only a few of metals occur free in nature. 9. At boiling point, water turns into vapour. 10. The students remained silent. 11. The hypothesis holds true under different conditions. 12. The solution turns yellow on standing. 13. He felt sorry for what he had done. 14. The predictions proved right. 15. It was 5 p. m. and the classes were over. 16. Dalton's atomic theory became one of the foundations of modern chemistry.
Ex. 11. Translate the sentences into English.
1. Кремний—один из самых распространенных элементов на земле. 2. Кремний встречается главным образом в виде диоксида кремния. 3. Кварц—это твердое бесцветное вещество. 4. Твердость кварца объясняется его структурой. 5. Структура кварца тссно связана со структурой кремнскислоты. 6. Кремнскислота — очень слабая кислота, она легко подвергается конденсации. 7. В кремнскислотс кремний окружен четырьмя атомами кислорода и к каждому атому кислорода присоединен атом водорода.
Ex. 12. Make up two or three questions to the italicized parts of the sentences.
1. Quartz crystals arc identified as right-handed or left-handed (2). 2. The structure of quartz and of other forms of silica is described as consisting ofSi04 tetrahedra (3). 3. In order to break a crystal of quartz it is ncccssary to break some silicon-oxygen bonds (2).
Ex. 13. Answer the following questions:
1. In what state docs silicon occur? 2. What arc the physical properties of quartz? 3. How is the hardness of quartz accounted for? 4. What do you know about silicic acid? 5. Describe the molecular structure of SiO*.
Section П
Упр. 1. Прочтите заголовок текста 7 В. Скажите, знаете ли вы что- ннбудь по этому вопрос}'.
Упр. 2. Назовите значения следующих интернациональных слов:
essential, primitive, organism, normal, laboratory, calcium, associate, mineralization, physiological, function, phosphorus, vitamin, vanadium, cffcct, pigmentation, observation, support, effective, litre, evolution, skeletal, role, toxicity
Упр. 3. Определите значения выделенных слов по контексту. Синонимы в скобках помогут вам.
1. Until now there has been no proof (evidcncc) for the importance of silicon in the life of animals or men. 2. Silicon is required (necessary) for normal growth of living beings. 3. Previous (early) laboratory studies showed several ways of obtaining hydrogen. 4. In the earliest stages (steps) of calcification in bones their calcium content (amount of calcium) is vciy low. 5. When a reaction proceeds quickly, we usually say that its rate is high. 6. The symbols for magnesium and fluorine arc Mg and F. 7. Other observations support the previous conclusion that silicon is essential. 8. Silicon is present in animal matter in small quantities. 9. Two is the average between 1 and 3.
Слова к тексту:
vital — жизненный; except — кроме; suggest — наводить на мысль; bone—кость; especially—особенно; plant—растение; egg—яйцо; bird — птица; appreciable — ощутимый; implications (pi) — толкования; participant—участник
Text 7 В
Прочтите текст про себя (контрольное время чтения — б минут). Silicon: an Essential Element for Life Processes
Until now there has been no proof that silicon plays any definite role in vital processes in animals or men. Scientists believed that it was nonessential except in certain primitive organisms. But later it was shown that silicon is required for normal growth and development of living beings.
Previous laboratory studies had suggested a possible role for silicon in bone formation, especially in young bone. In the earliest stages of calcification in bones, when their calcium content is very low, there is a direct relationship between silicon and calcium. Silicon is associated with calcium and increases the rate of bone mineralization. A relation has also been established between silicon, magnesium and fluorine in the formation of bones.
Some studies have also suggested the possibility that silicon has a physiological function. In addition to calcium, phosphorus, magnesium, iron and certain vitamins, silicon, along with tin, vanadium, and fluorine, has an effect of pigmentation.
Other observations support the previous conclusion that silicon is essential. The level of silicon effective for normal growth and development is of the order that is present in plant and animal food-stuff. Silicon is present in animal matter. The eggs of birds and milk have small but appreciable quantities. The blood of man averages about 5 mg of silicon per litre.
The discovery of the essential role of silicon in life processes has many implications, first, from an cvolutionaiy point of view, since silica performs a skeletal role in some primitive organisms, and, second, because, although great importance has been attached to the study of toxicity of silica, it has also been found that silicon itself can be considered as an important participant in normal metabolism.
Упр. 4. Скажите, узнали ли вы что-нибудь новое о кремнии. Что именно?
Упр. 5. Разделите текст на тематические части и озаглавьте каждую часть.
Упр. б. Скажите, что говорится в тексте:
1) о роли кремния в жизни живых организмов; 2) о физиологической функции кремния; 3) о том, какое количество кремния необходимо для нормального роста и развития; 4) о функции кремния в образовании костей.
Упр. 7. Найдите в тексте и переведите на русский язык предложения, где привадятся толкования роли кремния в жизненных процессах.
Section Ш
| Examples: |
Ex. 1. Think of the situations when the following expressions may be used: Vd like to...; what about you; the same to you
1. I'd like to go to the cinema today, and what about you? 2.1 wish you the best of everything.—The same to you.
Ex. 2. Translate the following into English. Let your fellowrstudents respond to your statements or questions. Use the expressions from exercise 1.
1. Я еще не знаю расписание на следующую неделю, а ты? 2. Мне хотелось бы сегодня поработать, давай встретимся завтра. 3. Завтра выходной, желаю тебе хорошо отдохнуть. 4. Я никогда не знал, что в молоке есть кремний, а ты? 5. Он хотел бы сдать экзамен завтра, б. Петров сдал уже все экзамены, а ты? 7. Лекции профессора Николаева очень интересны, я хотел бы записывать их полностью, но я пишу медленно. 8. Мне хотелось бы понять, почему кварц такой твердый. 9. Желаю тебе хорошо сдать все экзамены.
Ex. 3. Make up sentences or short stories with the following words:
1) dioxide, nature, three, crystal, silicon, occur, different, form; 2) be, widespread, silicon, quartz, most, mineral; 3) quartz, structure, hardness, mineral, account for; 4) play, important, silicon, vital, role, animal, men, process; 5) be present, silicon, appreciable, food-staff, amount
Ex. 4. Give detailed answers to the following questions:
1. In what form does silicon occur in nature? 2. What is the structure of quartz; what are the properties of this mineral? 3. What is the structure of silicic acid; what arc the properties of this acid? 4. In what living processes in animals or men docs silicon take part?
Ex. 5. Discuss the following topics:
1. An Abundance of Silicon.
2. The Structure of Si02 Molecule.
3. Properties and Structure of Quartz, Cristobalitc and Tridymitc.
4. The Role of Silicon in Living Processes.
DO YOU KNOW THAT...
Although the compounds of silicon have been used for many centuries, the clement was prepared only at the beginning of the 19th century. At present, there arc several methods of obtaining silicon. One of them is to heat silicon dioxide with magnesium:
Si02 + 2Mg = Si + 2MgO.
One way of preparing silicon commercially is by reducing Si02 with carbon in an electric furnace.
Lesson 8
ГРАММАТИКА: Составное глагольное сказуемое. Формы инфинитива и их значения. Модальные глаголы и их заместители.
Section I
Ex. 1. Pronounce the following words:
|
b) nevertheless [.ncvodo'les], giant ['d3aiont], tellurium [to'ljuoriom], selenium [si'lirniom], variety [vo'raioti], curious ['kjuorios], conductivity [.kondAk'tiviti], colloid ['kobid], gelatin ['dsclotinj, hydrazine ['haidro,zi:nj, hydrate fhaidreit], suspension [sos'penfn]
Ex. 2. Read the following words and say what Russian words help to understand their meaning:
elementary, interesting, giant, valence, allotropic, characteristic, variety, electrical, condition, colloid, gram, gelatin, hydrazine, hydrate, minute, observe, protective, serve, suspension
Ex. 3. Pay attention to the following way of word-building:
red + -is/i -» reddish (красн-ыи + -оват(ый) e красноватый) greenish, bluish, yellowish, pinkish, brownish
основа слова + -ful -> прилагательное
useful, beautiful, helpful, careful, truthful, thankful, peaceful, successful основа слова + -siort [fn], [311] -> существительное conversion, suspension, inversion; division, explosion, corrosion
Ex. 4. Arrange the following words in the alphabetic order and find their meaning in a dictionary:
textbooks, nevertheless, ores, infer, alike, picccs, coating, flame, peculiar, curious, varies, prolong
Ex. 5. Find the meaning of the words or expressions with the italicized words in a dictionary and translate the sentences into Russian.
1. Selenium is well worth studying. 2. It is worthwhile reading this textbook. 3. We compared the atomic weights of many elements.
4. Selenium has an atomic weight of78.96 as compared with 32 for sulphur.
5. His respect to his teachcrs was great. 6. In this respect selenium differs from sulphur.
Text 8 A Selenium
The clement selenium usually receives scant[3] attention in elementary textbooks, probably becausc it is of little importance commercially. Nevertheless, it is an interesting substancc and well worth studying.
Selenium was discovered by the Swedish giant among chemists, Bcrzclius.
The element is not abundant, but it is to be found in various ores.
Selenium is the sister element of sulphur, forming with tellurium the elements occurring in Group VI. It has an atomic weight of 78.96 as compared with 32 for sulphur. From the fact that the atomic weight is more, we may infer that selenium should be less active than sulphur. Its valences arc: +2, +4, and +6, the same as those of sulphur. It can be found in several allotropic forms, just as sulphur docs. It will be helpful to remember that the two elements are very much alike in their chemical properties and so the reactions of sulphur arc similar to those of selenium.
A piece of amorphous selenium is rather hard and quite brittle, just as sulphur is. The dark colour of the clement, the silver-grey coating on its surface arc characteristic. Another variety of the element is red.
The clement is both odourless and tasteless. It burns as readily as sulphur docs, with a reddish-blue flame and the peculiar odour. In working with selenium, beware[4] of the odour of its hydrogen compound; it is worse than that of hydrogen sulphide.
One curious property of selenium should be mentioned. The substance varies in its electrical conductivity according to the amount of light that falls upon it. We should remember that sulphur is a nonconductor. An experiment shows that selenium differs in this respect.
Under proper conditions selenium can form a colloid. One gram of selenium dioxide is dissolved in 500 ml of water. To 50 ml of this solution we add, after heating, 10 ml of a one-percent solution of gelatin, and then, drop by drop,* 60 ml of hydrazine hydrate (1:2,000 of water). We must remember to keep it just below the boiling point for 16 minutes. The beautiful pcach-pink" colour of the colloid is to be observed. The colloid can be made without gelatin, but the protective colloid serves to prolong the life of the colloidal suspension.
Words and Word-Combinations to Be Memorized
according to, alike, be alike, allotropic, amorphous, another, coating, colloid, compare, as compared, condition, conductivity, conductor, differ, drop, electrical, elementary, fall, few, a few, gram, heat, hydrate, importance, keep, mention, nevertheless, ore, piccc, proper, quite, rather, readily, in this respect, selenium, several, silver, sulphur, sulphide, surface, suspension, tellurium, textbook, variety, vary, be worth (while).
Ex. 6. Give the Russian equivalents for the following:
elementary textbooks, receive attention, be of importance, probably, nevertheless, bccausc, be worth studying, among, in various ores, the sister clement, as compared with, be less active, be more active, several allotropic forms, just as, remember, be very much alike, be similar to, amorphous
| i |
selenium, piece, rather hard, dark colour, die silver-grey coating, on the surfacc, another variety of, both... and..., burn readily, be worse than, mention, clcctrical conductivity, according to, fall upon smth., remember, be a conductor, be a nonconductor, differ in some rcspcct, under proper conditions, heating, a one-percent solution, drop by drop, the protective colloid, prolong the life
Ex. 7. Give the English equivalents for the following:
вероятно, иметь большое промышленное значение, тем не менее, интересный, срсдн химиков, в различных рудах, атомный вес, по сравнению с, быть более (менее) активным, такая же валентность, несколько аллотропных форм, быть полезным, быть очень похожим, довольно твердый, темный цвет, покрытие на поверхности, как... так и..., при работе с, в соответствии с, непроводник, в этом отношении, при надлежащих условиях, однопроцентный раствор, защитное покрытие, продлить жизнь
Ex. 8. Fill in the blanks with prepositions where necessary.
in 9 under, of by
1. Selenium is ... little importance commercially. 2. It was discovered ... Bcrzclius. 3. This element is found... various ores. 4. The valences ... selenium arc the same as those ... sulphur. 5. It can be found ... several allotropic forms. 6. It is helpful to remember that selenium and sulphur arc very much... alike... their chcmical properties. 7.... proper conditions selenium can form a colloid.
Ex. 9. a) Check up if you remember the following:
few—немногие, немного, мало; a few—несколько
употребляются с исчисляемыми существительными
тов в этой группе хорошо читают по-английски. 10. Вы мало работаете, вам надо работать больше.
с) Fill in the blanks with/ен', a few, little, a little.
1. Only ... information is available about iodine pcntafluoridc as a solvent. 2.... drops of concentrated HN03 were added to a decomposing melt containing chloride. 3. There is ... possibility that such ions can be produced under these conditions. 4. ... very interesting reactions have been shown at the lecture. 5. There are ... published papers on the preparation and properties of inorganic deuterium compounds. 6. High- tcmpcraturc reactions of polonium have been ... studied. 7. There can be ... doubt that the term "chemical structure*' was used for the first time in 1861 by Butlcrov. 8.... of these results have been reported previously.
Ex. 10. Translate the sentences into Russian, paying attention to modal verbs.
1. Selenium may be found in various ores. 2. We are able to describe the properties of any clement looking at the periodic table. 3. Selenium can occur in several allotropic forms. 4. Chemists must remember that sulphur is a nonconductor of electricity. 5. Under proper conditions wc may obtain a colloid. 6. He must be able to explain the difference between organic salts and inorganic salts. 7. Students have to understand reactions well. 8. Matter and its transformations must be studied by specialists. 9. Working in our laboratory, wc can change the state of substances. 10. The experiment is to be started at once. 11. You needn't heat the substances, the reaction proceeds fast enough. 12. You should know the properties of the substances if you have to work with them. 13. Sometimes we needn't accelerate the reaction. 14. Mendeleyev was able to predict in advance the existence and properties of yet undiscovered elements. IS. Mendeleyev couldn't be present at the meeting of Russian chemical society and had to ask Menshutkin to read the paper for him. 16. The "zero" group could be added to the periodic table only after the discovery of inert gases. 17. We should clean the glassware before working with it. 18. Lavoisier was able to establish his theory of combustion on the basis of the experimental results of Priestley, Sheclc and others. 19. The hydrides can decompose in water, releasing hydrogen. 20. You should remember that the yield in this reaction is good only if it goes to completion. 21. Students will be able to identify substances after some practice in qualitative analysis.
Ex. 11. Translate the sentences into English.
1. Селен не имеет большого промышленного значения. 2. Хотя селен не очень широко распространен, его можно найти в различных рудах. 3. Мы можем сказать, что атомный вес селена почти в два раза выше по сравнению с серой. 4. Из его положения в периодической таблице мы можем сделать вывод, что селен должен быть менее активным, чем сера. 5. Сера и селен очень похожи, и потому реакции серы похожи на реакции селена. 6. Селей без запаха и без вкуса, но может иметь несколько вариации по цвету. 7. При работе с сслсном следует помнить, что у его соединения с водородом запах хуже, чем у сероводорода. 8. Удельная электропроводность селена может изменяться в соответствии с количеством падающего на него света...
Ex. 12. Make up questions to the italicized parts of the sentence.
1. Selenium was discovered by the Swedish chcmist Bcrzclius in 1817 (4). 2. A piece of amorphous selenium is rather hard and quite brittle, just as sulphur is (2). 3. The protective colloid can prolong the life of the colloidal suspension of selenium (3).
Ex. 13. Answer the following questions:
1. Why is there only little information about selenium in elementary textbooks? 2. Why are the properties of selenium and sulphur alike? 3. What arc the physical properties of selenium? 4. What can you say about clcctrical conductivity of selenium and sulphur? 5. In what way can wc prepare a colloid with selenium? 6. How can the life of this colloid be prolonged?
Section П
Упр. 1. Прочтите заглавие текста 8 В. О чем, по вашему мнению, будет идти речь в этом тексте?
Упр. 2. Назовите значения следующих интернациональных слов:
metal, sclcnidc, proportion, material, action, chlorine, permanganate, cadmium, cobalt, nickcl, zinc, product, proccss, contain
Упр. 3. Определите значения выделенных слов по контексту. Синонимы в скобках помогут вам.
1. The atomic weight of selenium is approximately (about) 79.2. When H2SO4 is to be diluted with water, it must be poured into an excess (greater amount) of water. 3. Some reactions with conccntratcd sulphuric acid proceed quickly when they arc heated. 4. When sclcnious acid is treated with (actcd upon by) chlorine, sclcnic acid is produced. 5. When hydrogen sclcnidc forms, it bubbles (passes) into the liquids in the various bottles (containers for liquids). 6. The symbols Pb, Си, K, As and Sb stand for lead, copper,potassium, arsenic and antimony. 8. We obtained the product somewhat (to some extent) heavier than in the previous reaction.
Слова к тексту:
grind — измельчать; powder — порошок; mortar — ступа; dust — пыль; dry — сухой; vigorously — бурно; remove — удалять; lump — кусок; transfer — перемещать; flask — колба; connect — соединять; flue — газоход
Text 8 В
Прочтите текст про себя (контрольное время чтения — 5 минут). Selenium Compounds
The clement (selenium) can combinc with the metals Just as sulphur can, forming selenides. Let us grind some selenium to powder in a mortar. After that, wc should take 10 grams of this powder and grind it with 7 grams of iron dust, a proportion of 80 and 56, the approximate atomic weight ratio of the two elements. Then, wc must pour the well-mixed material into a dry test-tube and heat it. Ferrous scicnidc is formed, the action proceeds somewhat less vigorously than that between iron and sulphur. When the tube has cooled somewhat, wc can break it open and remove a grcy-black product which much resembles ferrous sulphide. Selenium will unite similarly with zinc and with other metals such as copper and lead.
Selenium dioxide may be formed by the combustion of the clement. The dioxide is white and can dissolve readily in water to form sclcnious acid just as sulphur dioxide dissolves to form sulphurous acid:
H20 + Sc02 -> H2ScOj.
When sclcnious acid is treated with chlorine or potassium permanganate, sclcnic acid is produced; the process, of course, can be called oxidation, the reaction proceeds just as it does with sulphurous acid. Small lumps of ferrous selcnidc arc transferred to a small flask connected to a train of bottles containing solutions of a number of different metallic salts, for example, those of cadmium, cobalt, nickel, arsenic, and antimony. Any excess of gas which may escape is led into the flue. Add some hydrochloric or sulphuric acid to the material in the flask, and presently hydrogen selenidc forms and bubbles into the liquids in the various bottles. Various selenides can be formed.
Упр. 4. Действительно ли текст 8 В содержит ту информацию, которую вы ожидали в нем найти? Скажите, что было для вас новым.
Упр. 5. Разделите текст на тематические части и озаглавьте каждую часть.
Упр. б. Скажите, что говорится в тексте:
1) о способности селена реагировать с металлами; 2) о реакции селена с железом; 3) об образовании диоксида селена; 4) об образовании селенистой кислоты; S) о получении селеновой кислоты.
| I |
Упр. 7. Найдите в тексте отрывок, nie описывается получение селе- нида водорода; переведите его на русский язык.
Scction III
| Examples: |
Ex. 1. Think of the situations when the following expressions may be used: be going to; be worth (while) + существительное или герундии; be used to
1. Pm going to finish this work at home.
2. Thc book is worth reading, but it is difficult to get it.
3. Нс is used to doing everything quickly.
Ex. 2. Translate the sentences into English, using expressions from exercise 1 •
1. Я не собираюсь делать домашнее задание сегодня. Уже поздно. 2. Я не буду заниматься иностранным языком регулярно. 3. Она привыкла вставать рано. 4. Есть студенты, которые привыкли получать хорошие оценки. 5. Он начнет реакцию заново, реагент был недостаточно чистый, б. Она говорила, что собирается на этот фильм. 7. Мы не привыкли переводить текст без словаря. 8. Стоит научиться писать сразу без ошибок, не придется тратить время на переписывание. 9. Он не привык к таким запахам. 10. Свойства некоторых соединений стоит запоминать. 11. Ссйчас эту статью читать не стоит, она будет для вас трудна.
Ex. 3. Make up sentences or short stories with the following words:
1) element, importance, abundant, little, commercial, selenium, be; 2) selenium, alike, property, atomic sulphur, be, weight, greater; 3) can, several, form, selenium, find, allotropic; 4) odourless, selenium, be, both... and..., tasteless, in colour, or, hard, red, dark, may; 5) metal, selenium, combinc, form, can, sclcnidcs
Ex. 4. Give detailed answers to the following questions:
1. What arc the physical properties of selenium? 2. What do you know about chemical properties of selenium? 3. In what respect arc selenium and sulphur alike? Why? 4. What selenium compounds do you know? 5. What methods of the preparation of selcnides do you know?
Ex. 5. Discuss the following topics:
1. An Abundance of Selenium and Its Compounds.
2. The Similarity of the Properties of the Elements Occurring in Group VI.
3. The Description of the Properties of Selenium.
4. Selenium Compounds.
DO YOU KNOW THAT...
In 1817 Bcrzclius managed to obtain a brown-red powder. It burned like sulphur. He established that it was a new element. He remembered the
other element with the similar properties — tellurium which has been given its name in the honour of die earth (from Greek "tcllus" meaning "earth"). He decided to name the newly-discovered clement in honour of the moon, that is why it is called selenium.
Lesson 9
ГРАММАТИКА: Выражение вероятности действия при помощи модальных глаголов.
Section I
Ex. 1. Pronounce the following words:
| e | [g] | go, green, agree, flag, game, garden, glass, good, great, grey, grow, gram, gas, group, organic, negative |
| g + e,!»У | [d3] | agent, gentle, g/ant, age, change, general, arrange, genius, hydrogen, halogen, oxygen, large, gymnasium, gyration, gvpsum |
| but: | [g] | give, get, girl, together |
| ng | [0] | long, strong, among, young, interesting, coating, according, prolong |
| nk, nc | № | pink, drink, think, thank, distinct |
| qu | [kw] | question, quick, quiet, quarter, quite, quartz, require, liquid, equipment, quantity |
| que | M | u'nique, technique |
b) chlorine [kb:'nn], hydrochloric ['haidrou'klonk], iodatc ['aiodeit], iodine ['aiodi:n], alcohol faelkohol], halogen [ЧкеЫзэп], saltpeter ('so:lt,pi:ta], violet ['vaioht], triiodide [trai'aiodaid]
Ex. 2. Read the following words and say, what Russian words help to understand their meaning.
halogen, unique, molecule, chlorine, manufacture, electrolysis, chloride, bromine, effect, agent, iodine, ion, iodatc, concentrate, lustre, violet, chloroform, indicate, alcohol
Ex. 3. Pay attention to the following way of word-building:
основа слова + -ine [i:n] -> существительное, глагол chlorinc, bromine, iodine, hydrazine, examine, determine
основа слова + -we [am] —> прилагательное crystalline, alkaline
| b) |
dis- + основа слова —> придает отрицателыюе значение, указывает на лишение, отделение, разделение discover, dissolve, disappear, disperse, disadvantage, disagreeable, disintegrate
Приставки, обозначающие количество
|
Ex. 4. Determine what part of speech the following words belong to and find their meaning in a dictionary.
undergo, touch upon, irritate, volatile, disagreeable, saltpeter, lustre, pain, sore, spill, skin, throat, Chile, tincture, hydrochloric, treat with, brine, gentle, keep
Ex. 5. Define the meaning of the italicized words.
1. Oil is callcd petroleum by the English. 2. Olive oil is used for cooking.
3. Iodine is ah almost black crystalline solid with a slightly metallic lustre.
4. A beautiful lustre was hanging in the room. 5. Men arc usually stronger than women. 6. A strong solution of sodium chloride was prepared.
Text 9 A The Halogens
Halogens may have interested chcmists sincc early times, for they possess unique properties. They must have interested scientists as all of them, F2, Cl>, Br2 and I2, consist of diatomic molcculcs. We arc going to touch upon some of their properties in what follows.
| a) |
Chlorinc (from Greek "chloros", greenish-yellow), the most common of the halogens is a greenish-yellow gas with a sharp irritating odour. It
was first made by the Swedish chemist K. W. Scheele in 1774, by the action of manganese dioxide on hydrochloric acid. It is now manufactured on a large scalc by the clcctrolysis of a strong solution of sodium chloride.
The element bromine (from Greek "bromos", stench) occurs in the form of compounds in small quantities in scawatcr and in natural salt deposits. It is an easily volatile, dark rcddish-browrn liquid with a strong disagreeable odour and an irritating effect on the eyes and throat. It may producc painful sores when spilled on the skin. The free element can be made by treating a bromide with an oxidizing agent, such as chlorine.
The element iodine (from Greek "iodes", violet) occurs as iodide ion, 1% in very small quantities in scawatcr, and, as sodium iodatc, NalOj, in deposits of Chile saltpeter. It is made commercially from sodium iodatc obtained from saltpeter, from kelp, which concentrates it from scawatcr, and from oil-well brines.
The free element is an almost black crystalline solid with a slightly metallic lustre. On gentle wrarming it gives a beautiful blue-violet vapour. Its solutions in chloroform, carbon tetrachloride, and carbon disulphidc arc also blue-violet in colour, indicating that the molecules I2 in these solutions closely resemble the gas molecules. The solutions of iodine in water containing potassium iodide and in alcohol (tincture of iodine) arc brown; this change in colour suggests that the iodine molecules have undergone chemical reaction in these solutions. The brown compound KI3, potassium triiodide, is present in the first solution, and a compound with alcohol in the second.
Words and Word-Combinations to Be Memorized
alcohol, bromine, chloride, chlorine, common, diatomic, follow, be going to, halogen, hydrochloric acid, iodide, iodine, lustre, manganese, oil, possess, potassium, producc, quantity, on a large scalc, sharp, sodium, suggest, tincture, touch, treat, unique, volatile
Ex. 6. Give the Russian equivalents for the following:
sincc early times, interest smb., possess unique properties, diatomic molecules, be going to, greenish-yellow, a sharp irritating odour, hydrochloric acid, be manufactured on a large scale, a strong solution, occur in the form of compounds, a small quantity, natural salt deposits, be volatile, treat smth. with an oxidizing agent, a black crystalline solid, a slightly metallic lustre, on warming, resemble gas molcculcs, undergo chemical reaction, be present in solution
Ex. 7. Give the English equivalents for the following:
галоген, состоять из двухатомных молекул, соляная кислота, в больших масштабах, электролиз раствора, раствор хлорида натрия, в морской воде, быть летучим, обладать уникальными свойствами,
3 Степаном 65 окислитель, встречаться, в очень малых количествах, получать из морской воды, элемент в свободном состоянии, крнсталличсское твердое вещество, металлический блсск, раствор йода в спирте, подвергаться реакции, находиться в растворе, предполагать (наводить на мысль)
Ex. 8. Fill in the blanks with prepositions where necessary.
in, of, with, on, by
1. All the halogens consist ... diatomic molecules. 2. The most common ... the halogens is chlorinc. 3. Chlorine was first made ... the action ... manganese dioxide... hydrochloric acid. 4. It was obtained by K.W. Schcelc ... 1774.5. Now chlorinc is manufactured ... a large scalc ... the electrolysis ... a strong solution ... sodium chloride. 6. Bromine occurs... the form of compounds ... small quantities. 7. Bromine has an irritating effect ... the eyes and throat. 8. Iodine is an almost black crystalline solid ... a slightly metallic lustre. 9. The I2 molecules ... the solutions ... chloroform, carbon tetrachloride and carbon disulphide closcly resemble the gas molcculcs. 10. The solution iodine ... alcohol is brown... colour.
Ex. 9. a) Check up if you remember the following:
much гораздо
r сравнительная степень
far + r намного
a great deal прилагательного —» значительно
stUl или наречия ^
much darker, far less abundant, still harder, a great deal more, much lighter, much more beautiful, far less active
b) Translate the sentences into Russian.
1. The first solution of sodium chloride is much stronger than the second solution. 2. Fluorine is a great deal more active than the other halogens. 3. Iodine is much heavier than bromine. 4. A surfacc coating protects some compounds from still further oxidation. 5. Potassium is much lighter than rubidium, and sodium is still lighter. 6. Amorphous boron is much more reactive chemically than is the harder, more expensive crystalline variety. 7. The nucleus of an atom is much smaller than is the atom itself. 8. Antimony is much more metallic in appcarancc and in properties than either phosphorus or arsenic. 9. Studies of crystal chemistry have attracted much greater interest during the last decade than ever before. 10. Carbon tetrachloride is a liquid much more dense than water. 11. Bromine is far less abundant than chlorine or fluorine. 12. Steel is far less brittle than cast iron.
Ex. 10. Translate the sentences into Russian, paying attention to modal verbs.
1. The text that you must have read describes the properties of halogens. 2. This student can't have started the reaction before learning the properties of the substances. 3. The author may have supposed that this description would help to understand his idea better. 4. The teacher may have spoken about such reactions, I don't remember. 5. Mendeleyev could have presented his periodic system himself, but he was ill. 6. You ought to have been more attentive working in the laboratoiy. 7. The scientists of our faculty may have discovered some new properties of electrodes, now they arc writing an article on this problem. 8. Everyone must have noticed a blue-violet vapour when iodine was being warmed. 9. The reaction may not proceed to completion without heating. 10. The laboratoiy assistant can't have done all the work alone. 11. Our friends haven't come yet, they must be still working in their laboratory. 12. You should have paid more attention to a theoretical course before starting your practice. 13. The reaction must have occurred, the colour of the reagents has changed. 14. Chcmistiy couldn't have reached the present level of development without the atomic thcoiy. IS. The discovery of the periodic law must have been the greatest discovery in the nineteenth-century chemistry. 16. The researchers would have tested their results by experiment, but they had no all the necessary apparatus. 17. Newton may have thought that light was a stream of particles. 18. Suggestions were made as to what may have occurred during the reaction.
Ex. 11. Translate the sentences into English.
1. Галогены—это элементы VII группы. 2. Все галогены состоят из двухатомных молекул. 3. Самый обычный из галогенов, хлор, — это зеленовато-желтый газ. 4. Хлор был впервые получен шведским химиком Шееле в 1774 году. 5. Сейчас хлор получают в больших масштабах электролизом раствора хлорида натрия, б. Бром встречается в малых количествах в виде соединений в морскойаоде и природных отложениях солей. 9. Йод также может встречаться в очень малых количествах в морской воде.
Ex. 12. Make up questions to the italicized parts of the sentences.
1. Halogens may have interested chemists since early times (3). 2. Iodine is an almost black crystalline solid with a slightly metallic lustre (3). 3. The solutions of iodine in water containing potassium iodide and in alcohol are brown (3).
Ex. 13. Answer the following questions:
1. What elements arc called halogens? 2. Why may the halogens have interested chemists? 3. What is the most common of the halogens? 4. When was chlorine obtained? 5. In what way is chlorine obtained now? 6. What kind of element is bromine? 7. Where does bromine occur and in what form? 8. In what way is iodine made commercially? 9. What kind of element is iodine?
Scction II
Упр. 1. Прочтите заглавие текста 9 В. Скажите, о чем, по вашему мнению, в нем пойдет речь? Что вы знаете об этом элементе?
Упр. 2. Назовите значения следующих интернациональных слов:
halogen, inert, react, reaction, rcactivc, reactivity, gas, electronegativity, asbestos, silicatc, aluminium, platinum, attack, container, protect, electrolysis, electron, electrodc, limit, voltage, extremely
Упр. 3. Определите значения выделенных слов по контексту. Синонимы в скобках помогут вам.
1. The symbols for fluorine and copper arc F and Cu. 2. Fluorine forms compounds with all the elements except the lighter inert gases. 3. Hold (keep) the test-tube in hot water. 4. Even asbestos reacts very vigorously (intensively) with fluorine. 5. The substancc is called incandescent when it is heated so much that it is giving out light. 6. Chemists expect (believe, hope) that some new elements will be synthesized. 7. The oxidizing power (ability to oxidize) of an electrode can be increased.
Слова к тексту:
attribute to—относить к; wood—древесина; rubber — каучук, резина; burst into flame — загореться; stream — поток; thin — тонкий; layer — слой; affinity — сродство; it took him — ему потребовалось
Text 9 В
Прочтите текст про себя (контрольное время чтения — 4 минуты).
Fluorine
Bccausc its electronegativity is greater than that of any other clement, we cannot cxpect that fluorine could be prepared by reaction of any other… have been extremely difficult, for it took him several years of hard… Упр. 4. Скажите, узнали ли вы что-ннб)дь новое из текста 9 В? Если узнали, то что именно?Lesson 10
Section I Ex. 1. Pronounce the following words: С + e, i,y M …Acctic acid, addition, almost, aluminium, analysis, anhydrous, approximately, attempt, available, basic, boil, case, collect, compose, composition, dilute, dircct, clcctrodc, equation, evaporate, exception, exhibit, extract, filter, fluoride, halide, hardly, hydroxide, indium, laboratory, lie, mass, material, most, nitric acid, oxidation, per cent, potential, precipitate, prevent, regard, residue, salt, seldom, scries, sulphite, soluble, supply, take placc, volt
Ex. 6. Give the Russian equivalents for the following:
attempt applications, regard smth. as, hardly, be available, immediate prospects, over 1 per cent, exhibit properties, burn with a blue flame, in the prcscncc of, take placc, in cach ease, oxidation state, with the exception of, be soluble in water, in the elcctromotivc series, an clcctrodc potential, dilute acid, react with the evolution of hydrogen, together, concentrated
acid, acctic acid, according to the equation, to have some effect on smth., to dissolve almost all the material, the resulting solution
Ex. 7. Give the English equivalents for the following:
в течение многих лет, едва ли возможно, в количестве свыше 1 процента, рассмотреть, несколько методов, разбавленная ссрная кислота, почти все вещество, на фильтре, получающийся раствор, проявлять характерные свойства, горсть на воздухе, прямое соединение, происходить (иметь место), в каждом случае, за исключением чего- то, в присутствии воды, безводная соль, большинство соединений, концентрированная кислота, по уравнению (согласно уравнению), спектральный анализ
Ex. 8. Fill in the blanks with prepositions where necessary.
of, at, in, between, with, from, according to, for
1.... many years indium was regarded as a laboratoiy curiosity. 2. There arc several methods ... extracting ... indium ... its ores. 3. ... one ... the methods... obtaining indium, indium-bearing zinc is treated... dilute H:S04. 4. Indium exhibits properties characteristic... the aluminium group. 5. Indium burns ... air ... a blue flame. 6. If indium is heated ... the presence ...
halogens or sulphur, direct combination takes place. 7 each case indium
is ... its highest oxidation state. 8. Only the halides, InX3, arc stable ... the presence ... water. 9. Most indium compounds arc soluble ... water. 10. Indium lies... iron and tin... the electromotive series. 11. Characteristic spectral blue lines ... indium occur... 451.1тц and 410.1 тц.
Ex. 9. a) Mind:
| Examples: |
No article is used before nouns denoting some branch of science, the names of chemical elements and the word school.
1.My younger sister goes to school.
2.While at school I liked chemistry best.
3. Indiumhas an clcctrodc potential of+0.336 volt.
b) Translate the sentences into English.
1. В университете мы изучаем математику, химию, физику, историю и английский язык. 2. Он не любит математику. 3. Она преподаст общую химию. 4. В школе мне правился английский язык. 5. Какие предметы тебе нравились в школе? 6. Какие оценки у тебя были по химии? 7. Фтор — самый интересный из галогенов, так как он самый активный. 8. Моя сестра ходит в школу, больше вссго ей нравится русская литература. 9. Индий не встречается в больших количествах. 10. Некоторые элементы, такие, как кальций, железо, магний, играют важную роль в жизни живых организмов. 11. В природе бром встречается реже, чем хлор и фтор.
Ex. 10. Translate the sentences into Russian, paying attention to the subjunctive mood.
1. There would be no life without such elements as carbon, oxygen, hydrogen, etc. 2. It is necessary that the substanccs be pure. 3. The teacher suggested that the equation of the reaction be written on the blackboard. 4. The solution is diluted lest it be very strong. 5. They would do it oncc more if they had time. 6. The condition was made that the reaction should procccd slowly. 7. He should have told us about the new time-table. 8. She should have done it yesterday but she didn't know that her results were not accuratc. 9. Heating or cooling would affect the state of water, but not its composition. 10. Dry gas would occupy less volume. 11. It would be difficult to find a more abundant element than oxygen. 12. The corrosion would procccd much more rapidly in the prcscncc of oxygen. 13. We would begin the calculation but wc have no all the ncccssary data. 14. You should have known that these arc inflammable substanccs. IS. They should have described the properties of the substanccs that were obtained. 16. It would be true to say that experiment is the foundation of chcmistry. 17. The new apparatus would have given more accurate results. 18. It is essential that water should be turned into vapour. 19.1 wish she were right. 20.1 should have asked the tcachcr but I could not come to the seminar. 21. They suggest that the experiment be started at once.
Ex. 11. Translate the sentences into English.
1. Индий встречается в природе в очень незначительных количествах. 2. Свойства индия характерны для элементов III группы. 3. Индии горит на воздухе и образует окисел 1п203.4. Большинство соединении индия растворимы в воде. 5. Разбавленная соляная кислота реагирует с индием с выделением водорода. 6. Уксусная кислота не растворяет индия. 7. Спектральный анализ показывает характерные для индия синие линии.
Ex. 12. Make up questions to the italicized parts of the sentences.
1. Indium lies between iron and tin in the electromotive scries (2).
2. Two reactions arc possible with concentrated sulphuric acid (3).
3. Several methods of extracting indium from its ores arc possible (2).
Ex. 13. Answer the following questions:
1. What is the occurrcncc of indium in nature? 2. What properties docs indium exhibit? 3. What can you say about commercial applications of indium? 4. What kind of oxide docs indium form? 5. What compounds of indium and the halogens arc known? 6. In what way docs indium react with sulphuric acid? 7. What docs the spectral analysis show?
Scction II
Упр. 1. Прочтите заглавие текста 10 В. Скажите, знаете ли вы что- IIII6)71 ь по этому вопросу?
Упр. 2. Назовите значения следующих интернациональных слов:
material, medicine, pharmaceutical, thallium, platinum, busmuth, temperature, corrosion, discomfort, patient, dentist, amalgamate, diffuse, uniform, porous, attack, diffuse
Упр. 3. Определите значения выделенных слов по контексту. Синонимы в скобках помогут вам.
1. Sometimes chemists add indium to obtain desirable (needed) properties of substances. 2. Indium forms alloys (mixtures of metals) with lead, tin, silver, gold, platinum and others. 3. Unique properties of indium enable (give the possibility) it to be used in medicine. 4. Indium alloys arc very corrosion-resistant (unaffected by corrosion). 5. Indium can be deposited on the article (Uiing) coated with zinc or cadmium.
Слова к тексту:
purpose — цель; jeweller}' — ювелирные изделия; mordant — вещество, закрепляющее краску; dyestuff— краситель; braze—паять; insert — вставка; cast — форма (для отливки); limbs — конечности; molten — расплавленный; filling — пломба (зубная); conventional — обычный; plate— покрывать металлом; bake — запекать; chip — откалываться; peel — шелушиться
Text 10 В
Indium finds most of its uses as an addition to other materials for the purpose of obtaining more desirable properties. Indium and its compounds also find uses in jewellery, pharmaceuticals,… Indium forms alloys with many metals; some of them arc lead, thallium, tin, silver, gold, platinum, mercury, bismuth,…Lesson 11
Scction I Ex. 1. Pronounce the following words. pb ra physical,…Fascinating Phosphorus
ТЪе history of phosphorus is no less interesting than the element itself. It was first prepared in 1669 by a German alchemist Hcnnig Brand, who like… Brand kept the details of the preparation of this strange substance secret,… Phosphorus may be fascinating, but is also intensely poisonous. It should never be allowed to touch the skin, as it…Modifications of Phosphorus
The phosphorus obtained by the reduction of phosphorus is always the yellow variety. This is "regular" phosphorus and the other allotropic… Phosphorus is able to combine readily with oxygen and ignite in the air at 30… Phosphorus is very soluble in carbon disulphide.Lesson 12
Section I Ex. 1. Pronounce the following words: Помните, что в многосложных словах ударение часто падает на 3-й слог от конца слова, а ударные гласные читаются…Chemical Symbols for Representing Compounds
A molecule of a compound is defined as the smallest part of a compound that can exist as a free and separate substance. For crystalline solids in… For gases, the molcculc is a perfectly real entity, and die molecular weight…Weight of 1 molcculc
Weight of У12 atom of carbon 12
Though the terms "gram-molecular weight" and "gram-atomic weight" are no longer used by chemists, it is interesting to know how… Упр. 4. Оправдались ли ваши предположения насчет отлнчня по содержанию текста… Упр. 5. Разделите текст на тематические части н озаглавьте их.Part Two THE SUBJECT
Lesson 13
Section I Ex. 1. Practise your pronunciation. a) period ['pionod], ancient ['einjont], civilization [.smlai'zeijn], China ['tjaino], India ['indjo], Greece [gri:s],…Text 13 A The History of Chemistry
The alchemical period (350-1500), of which the principal goals were an elixir of life and the philosopher's stone by which base metals could be… Iatrochcmists (1500-1650) devoted their chemical pursuits to alleviation of… When qualitative and quantitative analysis had identified enough pure substances, inorganic chemistry grew in scope.…Text 13 В
Twenty-first-ccntury chemistry has narrowed into units such as instrumental analysis, biochemistry, chemical engineering, and colloids. Chemistry… The great frontiers of our world and our universe that await the explorer and… 4 СтепаномLesson 14
Section I Ex. 1. Practise your pronunciation. a) thought [0o:t], continuous [kon'tinjoos], surfacc ['s3:fis], argue ['a:gju:], infinitely ['infimth], inquire…Text 14 A The Idea of the Atom
During the period of fourteen years beginning with 1897, it was discovered that atoms are composed of smaller particles. The discovery of the… Words and Word-Combinations to Be Memorized argue, believe, bring about, confirm, continuous, course, of course, fundamental, hold, individual, infinite, inside,…Text 14 В
The Greek philosopher Democritus (about 460-370 B.C.) who had adopted some of his ideas from earlier philosophers, stated that the Universe is… was pure speculation, and was much too general to be useful. Dalton's atomic… Dalton stated the hypothesis that elements consist of atoms, all of the atoms of one clement being identical9, and…Lesson 15
Section I Ex. 1. Practise your pronunciation. a) physicist [Tizisist], previously fprirvjosh], among [э'тлр], weight [weit], aggregate ['acgngat], encyclopaedia…Text 15 A The Atomic Theory
The rapid progress of scicncc during the twentieth ccntury is well illustrated by the increase in our knowledge about atoms. In a popular textbook… Only half a ccntuiy later, scientists had precise knowledge of the structure… Words and Word-Combinations to Be MemorizedText 15 В
Most our knowledge of the electronic structure of atoms has been obtained by the study of the light given out by atoms when they arc excited by high… The careful study of line spectra began about 1880. Early investigators made… Упр. 3. Передайте основное содержание текста в нескольких предложениях.Lesson 16
Section I Ex. 1. Practise your pronunciation. a) molcculc ["mohkju:!], peculiar [pi'kjuiho], rearrangement [,ri:o'rein- d3mont], neutral ['nju:trol], other…Text 16 A Molecules
In one type of union, atoms bccomc bonded together to form definite aggregates that exist as independent, electrically neutral particles and arc… To give a short definition of a molcculc is not to give a more or less full… Molcculcs arc regarded as the smallest particlcs or elementary substanccs that can have independent existence. They…Text 16 В
Прочтите текст про себя (контрольное время чтения—4,5 минуты).
Molecular Composition and Size
Not all molcculcs are molecular in structure. Some are atomic and many are ionic. Molecular substances are characterized by low boiling points and… The size of molecules, especially of the smaller ones, is so tiny that to make… A cup of water taken at random from your nearest supply, would give you at least one hundred of the original dyed…Lesson 17
ГРАММАТИКА: Герундий и герундиальный оборот в функции подлежащего.
I Section I
a) equal ['i.kwolj, pressure ['prcjo], consequence ['konsikwons], gaseous ['gasidsj, weight [weit], cohesion [кэо'Ы.зэп], adjacent [o'dseisont],… b) theu'samcu'numbcr^ofmoleculcs, atjhe^'sameNpressurc, through the ycais,… c) The 'molecules in a 'liquid | are 'not 'all 'moving at the 'same velocity.Text 17 A Molecules in Gases and Liquids
At the same temperature, molccules of a liquid move at the same rate as those in a gas. In a liquid, however, the extent of motion must be more… The molecules in a liquid, like those in a gas, are not all moving at the same… layer, have no force of attraction from molcculcs above. Some of the more rapidly moving molcculcs overcome the…Lesson 18
Scction I Ex. 1. Practise your pronunciation. a) iodine faiodirn], equilibrium [ ,i:kwi'libnom], fluidity [flu'iditi], viewpoint fvjurpoint], process fprouses],…Text 18 A The Nature of a Liquid
Liquid iodine differs from solid iodine (crystals) mainly in its fluidity. Like the solid, and unlike the gas, it has a definite volume (1 g… From the molccular viewpoint the proccss of melting can be described in the… Words and Word-Combinations to Be MemorizedText 18 В
That the molcculcs of a gas arc not held together, but arc moving freely in a volume rather large compared with the volumes of the molcculcs… Gases at ordinary pressure arc very dilute: the molcculcs themselves… Упр. 3. Передайте основное содержание текста в нескольких предложениях.Part Three THE OBJECT
Lesson 19
Section I Ex. 1. Practise your pronunciation. a) originally [o'ridsonoh], stereochemistry [.stena'kemistn], final [Tainol], postulate ['postjoht], draw [dn>:],…The Study of the Structure of Molecules
In the nineteenth century the valcncc bond was represented by a line drawn between the symbols of two chcmical elements, which expressed in a… following 1916, or in the book Valence and Structure of Atoms and Molecules… All of these early studies, however, contained, in addition to suggestion that have since been incorporated into the…Complcx, consideration, coordinate, dccadc, discard, discuss, draw, especially, essentially, formation, incorporate, lead to, line, numerous, originally, paper, phenomenon, with regard to, relate, and so on, stability, stable, structural, take part, valcnce, valuable
Ex. 5. Give the Russian equivalents for the following:
carry on the study, essentially chcmical in nature, take part in a reaction, from the consideration of facts, formulate the theory of valence, structural formulas for molcculcs, final form, tctrahcdral orientation of the bonds, represent the bond by a line, draw a line between, have qualitative significance, with regard to molecular structure, form the basis of a theory, share the electrons between the atoms, the features of the detailed theory, be incorporated into
Ex. 6. Give the English equivalents for the following:
изучение структуры молекул, методы исследования, первоначально, химический состав, существование изомеров, природа химической реакции, и так далее, принять участие в реакции, такого рода факты, столетие тому назад, написать структурную формулу, четыре валентные связи атома углерода, теория стереохимии, провести линию, многочисленные попытки, сделать попытку, развить теорию, электронная теория валентности, образование химической связи, ко- валентная связь, поделить электроны между чём-л., подчеркнуть значение чего-л., применение новых идей, предложить теорию, кроме, первые исследования
Ex. 7. Fill in the blanks with prepositions where necessary.
1. Early chemists carried ... the study ... the structure ... molcculcs ... the methods that were essentially chcmical ... nature. 2. The first structural formulas ... molecules were written ... more than a ccntury ago. 3. ... the 19th ccntuiy the valcnce bond was represented ... a line ... the symbols ... two chcmical elements. 4. The nature ... the bond was completely unknown. 5. ... the discovery ... the electron attempts were made to develop an electronic theory ... the chcmical bond. 6. ... 1916 Lewis formed ... the basis ... the modern electronic theory ... valence. 7. Early studies contained ... some suggestions that were discarded later.
Ex. 8. Translate the scntcnccs into Russian, paying attention to different functions of the word regard.
I. At the beginning of the 19th ccntuiy, atoms were regarded as the smallest constituent particles of matter. 2. Equal volumes of gases arc stated to contain the same number of molecules at the same temperature and pressure, regardless of composition. 3. Unfortunately, it's not always that the young have great regard to their parents. 4. Particular attention must be paid to the chapter regarding the conditions of synthesis. 5. Many chcmical facts were described which had only qualitative significance with regard to molecular structure. 6. The work of Lewis was most important as regards the development of the clcctronic theory of valcncc.
Ex. 9. Translate the sentences into Russian.
a) 1. These results arc not in agreement with the theory. 2. They did their work in time. 3. Register the readings and then begin the calculations.
4. Put the burner on the table. 5. The discovery of the electron stimulated the development of an clcctronic theory. 6. The information was given to a numerous audicncc. 7. The rate of the reaction often depends on the temperature. 8. The heating resulted in the explosion. 9. Give this instruction to the students. 10. Give the students this instruction.
11. Lowering the temperature of a liquid lessens the kinetic energy.
12. Hydrogen has 3 isotopes. 13. The study of the structure of molcculcs has been carried on by scientists since early times.
b) 1. Have you seen him? 2. Did you speak to her yesterday? 3.1 don't know anything about the author of this monograph. Do you know anything about him? 4. It is a very good apparatus, everybody likes to work with it.
5. The bottle with H2S04 is on that shelf, bring it, please. 6. The temperature must be 2S°C, don4 forget it. 7. They think it ncccssaiy to attend all the seminars. 8. The teacher made it clear that the examination will be difficult to pass. 9. Avogadro's principle let one calculatc the number of molcculcs per gram mole of a gas. 10. Chcmistiy helps one to understand other fields of scicncc better. 11. The properties of HC1 make it a useful laboratory reagent. 12. Consideration of all the facts enabled us to draw a very important conclusion. 13. They don't think it possible to work with this substancc, it is not pure enough.
Ex. 10. Translate the sentences into English.
1. Проблема структуры молекулы давно интересовала химиков.
2. Структурная формула — это формула, которая показывает, как оруппированы элементы и как распределяются связи в молекуле.
3. Бутлеров был первым из русских ученых, кто написал структурную формулу. 4. В XIX столетии валентную связь представляли линией, которую проводили между символами двух химических элементов. 5. Природа связсй тогда была совершенно неизвестна. 6. При изучении структуры молекул было открыто существование изомерии.
7. Изомеры — это соединения, которые имеют одну и ту же молекулярную формулу, но разные структуры.
Ex. 11. Answer the following questions:
1. What methods were used in the first investigations of the structure of molcculcs? 2. What do you know about isomers? 3. Who formulated the theory of valence? 4. What is particularly interesting as regards the valence bonds of the carbon atom? 5. In what way was the valence bond represented? 6. How did the discovery of the electron affect the development of an electronic theory? 7. What do you know about the work of Lewis?
Section II
Упр. 1. Назовите значения следующих интернациональных слов:
diffraction, magnetic, dipolc, moment, interpretation, resonance, entropy, information, configuration, quantum, mechanics, anticipate, discussion
Упр. 2. Определите значения выделенных слов по контексту.
1. Properties of an element are due to the composition and structure of its atom. 2. Our laboratory is provided with all the necessary chemicals and devices. 3. It is not always easy to give a complete characteristic of a compound. A. X-rays arc sometimes callcd Rontgcn rays. 5. In a molecular spectrum a scries of rather broad bands is formed, that is why it is called band spectrum. 6. It is necessary to take into account the unusual properties of the compound.
Слова к тексту:
with respect to — в отношении чего-л.; wave — волна; nuclear — ядерный; refinement — усовершенствование; disperse — рассеивать; veil — покров, завеса; mystery—тайна; shroud — окутывать
Text 19 В
Прочтите текст про себя (контрольное время чтения — 3 минуты). The Development of the Theory of Valence
Modern structural chemistry differs from classical structural chemistry with respect to the detailed picture of molecules and ciystals that it presents. By various physical methods, including the study of the structure of crystals by the diffraction of X-rays and of gas molcculcs by the diffraction of electron waves, the measurement of electric and magnetic dipolc moments, the interpretation of band spectra, line spectra, microwave spectra, and nuclear magnetic resonance spectra, and the determination of entropy values, a great amount of information has been obtained about the atomic configurations of molcculcs and crystals and even their electronic
structures; a discussion of valcncc and the chcmical bond now must take into account this information as well as the facts of chemistry.
The rcfinmcnt of the clcctronic theory of valcncc into its present form has been due" almost entirely to the development of the theory of quantum mechanics, which has not only provided a method for the calculation of the properties of simple molccules, leading to the complete elucidation of the phenomena involved in the formulation of a covalent bond between two atoms and dispersing the veil of mystery that has shrouded the bond during the decades since its existence was first assumed, but has also introduced into chcmical theory a new concept, that of resonance, which, if not entirely unanticipated in its applications to chemistry, nevertheless, has not before been clearly recognized and understood.
Упр. 3. Передайте основное содержание текста в нескольких предложениях.
Упр. 4. Составьте план текста.
Упр. 5. Прочтите предложения и скажите, соответствуют ли они содержанию текста. Если нет, исправьте их.
1. Structural chcmistry hasn't changed since the 19th century. 2. The pictures of molcculcs presented by modern chcmistry and classical structural chcmistry arc not the same. 3. Electronic structures arc investigated only by chcmical methods. 4. The development of the thcoiy of quantum mechanics affcctcd the refinement of the clcctronic theory. 5. A concept of resonance was introduced in terms of the theory of quantum mechanics. 6. Application of quantum mechanics enabled scientists to develop a method for the calculation of the properties of simple molcculcs.
Упр. б. Найдите в тексте описание различий между современной * структурной химией и классической и переведите эти предложения на русский язык.
Scction UI
Ex. 1. Respond to the following, using the expressions: to do smth (ito work, to study, to make observations, etc.) under smb (Prof. N, Dr. A, etc.).
1. The laboratory is now an important scientific ccntrc, who is the head of it? 2. He says he has a very good scicntific adviser, under whom docs he study? 3. He has written a very good article, whom did he consult? 4.1 know diat he docs physics at Moscow University, who is his supervisor? 5. You should consult your tcachcr before making observations, who is your tcachcr? 6. This book is rather difficult but very useful for your work, who advised you to read it? 7. Your brother obtained his degree last year, who was his scientific adviser?
Ex. 2. Translate the sentences into English.
1. Я выполнил эту работу под руководством профессора N. 2. Сту- дснчсскос научное общество работает под руководством доктора N. 3. Группа, которой руководит доктор N, недавно опубликовала очень интересную статью о своих последних исследованиях. 4. Под чьим руководством вы проходите практику? 5. Мы занимаемся в лаборатории качественного анализа под руководством N. б. Моя подруга выполняет свою курсовую работу под руководством N, ей очень нравится работать с ним. 7. Я не знаю, под чьим руководством мы будем работать в следующем учебном году.
Ex. 3. Make up short dialogues according to the model.
Model: — A question.
— An answer. (+, -)
— A request.
Example: — Are you going to the dean's office today?
— 1 think so.
— Could you give my application to the secretary?
Ex. 4. Give detailed answers to the following questions:
1. What can you say about the early studies concerning the structure of molcculcs? 2. What important discoveries were made in the course of structural investigations? 3. In what way was the valence bond understood in the 19th ccntury? 4. What investigations laid the foundation of the modern electronic theory? 5. What methods were used to study the structure of molcculcs?
Ex. 5. Discuss the following topics:
1. The Contribution of the Great Scientists of the Nineteenth Ccntury to the Structural Theory.
2. The Methods Used in Structural Investigations.
3. The Refinement of the Electronic Theory of Valcnce.
WHAT IS IT?
The combining power of an atom, equal to the number of hydrogen atoms which an atom will combine writh or replace.
Lesson 20
Section I Ex. 1. Practise your pronunciation. a) covalent [koo'veilont], distribute [dis'tribjot], electron [f lcktron], ethyl [cOilj, ['i:0ail] (chem. dimethyl…The Structure of Covalent Compounds
For many molcculcs the covalcncc of cach atom is equal to the number of unpaired electrons in its outer shell, and is, thus, simply related to the… It is often ncccssary to have some experimental information about the way in… The atoms of most molcculcs are held tightly together by a very important sort of bond, the sharcd-clcctron-pair bond…Covalcncc
""" When two or more atoms share electrons to form molcculcs, all the electrons of the several atoms involved[7] become common… The carbon-atom has two electrons in its first main energy level and four in… Упр. 3. Передайте основное содержание текста в нескольких предложениях.Lesson 21
Section I Ex. 1. Practise your pronunciation. a) require [n'kwaid], completion [kom'pli'Jon], extremely (iks'tri:mh], neutralization [,nju:trolai'zeiJon],…Factors Influencing the Rate of Reactions
Ag*(aq) + Cl-(aq) AgCl(c). On the other hand, ionic oxidation-reduction reactions arc sometimes very… гРс^ + Зп^гРс^ + Бп4'.A Catalyst
Catalysts arc usually specific in action; thus, a catalyst for one reaction is more often than not useless for any other reaction. They are… Chemists have discovered the great majority of reactions that proceed without… The phenomenon in which a relatively small amount of a foreign material, callcd a catalyst, augments the rate of a…Lesson 22
Section I Ex. 1. Practise your pronunciation. a) determine [df t3:min]f prevail [pn'veil], consequently ['konsikwontli], identify [af dentifai], inasmuch…Text 22 A Factors Affecting the Boiling Point
Each individual substancc capable of existing in the liquid state has its own definite boiling point. Consequently, this property is used for… Inasmuch as the boiling point of a substancc is affcctcd by changes in… of 760 mm Hg. Nevertheless, it is quite true to say that the boiling temperature of water is 70°C at a pressure of…Text 22 В
If two objects are placed in contact with one another, thermal energy may flow from one objcct to the other one. Temperature is the quality that… Temperatures are ordinarily measured by means of a thermometer, such as the… temperature of freezing water saturated with air is 0°C and the temperature of filing water is 100°C at 1-atm…Lesson 23
Section I Ex. 1. Practise your pronunciation. a) measure ['тезэ], centigrade ['scntigrcid], advance [od'va:ns], usage [ju:z)d5], bureau ('bjuorao], adopt [o'dopt],…Text 23 A Celsius versus Centigrade
"Celsius" appeared desirable, but it was not practicable to impose this term on those who preferred "centigrade". In preparation for the General Confcrcncc the National Bureau of Standard… With regard to the merits of the decision it might be remarked that Celsius (abbreviated C) was analogous to the names…Text 23 В
Прочтите текст про себя (контрольное время чтения — 4 минуты).
The Kelvin Temperature Scale and Modern Means of Measuring the Temperature
The International Standard temperature scalc is the Kelvin scale with a new definition of the degree. The absolute zero is taken to be OK and the…Gas Thermometer
Упр. 3. Передайте основное содержание текста в нескольких предложениях. Упр. 4. Составьте план текста. Упр. 5. Прочтите предложения и скажите, соответствуют ли они содержанию текста. Если нет, исправьте их.Lesson 24
Section I Ex. 1. Practise your pronunciation. a) recognize ['rckognaiz], relative ['relotiv], carefully ['kcofuli], determine [df t3:min], electrolysis…The Composition and Structure of Water
The formula of water is H20. The relative weights of hydrogen and oxygen in the substancc have been very carefully determined as 2.016:16.000. This… Liquid water has a number of unique properties which indicate that the… From spectroscopic studies of isolated water molcculcs in the gas phase, it has been shown that the H—О—H bond angle…Text 24 В
Прочтите текст про себя (контрольное время чтения — 4 минуты).
Water
One of the most striking properties of water is its ability to dissolve many substances, forming aqueous solutions. Solutions are very important kinds of matter — important for industry and for life. The ocean is an aqueous solution that contains thousands of components: ions of the metals and non-metals, complex organic ions, many different organic substanccs. It was in this solution that the first living organisms developed, and it was from it that they obtained the ions and molcculcs needed for their growth and life. In the coursc of time organisms that were evolved could leave this aqueous environment, and move out onto the land and into the air. They achieved this ability by carrying the aqueous solution with them, as tissue fluid, blood plasma, and intracellular fluids containing the neccssary supply of ions and molccules.
The properties of solution have been extensively studied, and it has been found that they can be correlated in large part by some simple laws.
Water not only is the most widely used of all solvents, but also, of all liquids, it most nearly approaches being the "universal solvent". Every substancc is probably soluble in water to some extent, although in many cases the solubility is so small that it is almost impcrscptiblc. Thus, a saturated solution of barium sulphate contains less than a quarter of a milligram of solute per litre, and the solubility of silicon dioxide (quartz)—is even smaller than this. Yet, water that is allowed to remain for a long time in a quartz vessel must eventually become saturated with quartz; a saturated solution of this substancc is so extremely dilute, however, that many litres of it would have to be evaporated to dryness in order to yield a visible residue. Such substanccs as barium sulphate, silver chloride, quartz, glass, mercury, and cellulose are usually considered as "insoluble" in water, but this term, it must be remembered, is merely a relative one, and it would be more accuratc to say that their solubility is exceedingly small.
Упр. 3. Передайте основное содержание текста в нескольких предложениях.
Упр. 4. Составьте план текста.
Упр. 5. Прочтите предложения и скажите, соответствуют ли они содержанию текста. Если нет, исправьте их.
1. Water can dissolve a great number of substanccs. 2. Solutions arc important in industry, but not in life. 3. A lot of components are dissolved in the occan water. 4. The first living organisms developed in water and on the land and then moved into the air. 5. There arc no laws common to all the solutions and the properties of cach solution arc studied separately. 6. Every substancc is soluble in water to some extent.
Упр. б. Найдите в тексте и переведите на русский язык предложения с описанием того, как ведут себя в воде вещества, которые считаются практически нерастворимыми.
Section III
Ex. 1. Respond to the statements and questions, using the following expressions: / hope so; so it is; it is; it would be wrong to say so; Vm of the same opinion.
1. I think wc speak too much about water, there arc many other interesting liquids. 2. Barium sulphate is insoluble in water. 3. The occan is an aqueous solution, isn't it? 4. It was in the occan that the first living organisms developed. 5. Could wc obtain a solution of quartz? 6. Thus, in conclusion wc may say that the term "insoluble" in water is not accuratc when wc speak about silver chloride. 7. Water is usually called the "universal solvent". 8. Soon wc shall learn the structure of water and then we shall clcarly understand the nature of its properties.
Ex. 2. Translate the sentences into English.
1. Тысячи компонентов различных веществ растворены в воде океана. — Да, это так. 2. Наверное, прошло много времени, прежде чем живые организмы вышли из океана на сушу. — Я тоже так думаю. 3. Свойства растворов хорошо известны, и мы тоже скоро будем их изучать. — Надеюсь. 4. Есть вещества, например, сульфат бария, которые не растворяются в воде. — Было бы неверно так говорить, потому что он растворяется, только очень мало. 5. Структура воды уникальна. — Да. 6. Теплоемкость воды необычно высока. —Да, это так.
Ex. 3. Make up short dialogues according to the model.
Model: — A request
— A clarifying question.
— A statement.
— An answer.
Example: — Could you help me with my homework?
— What is the difficulty?
— I can't understand this rule.
— I'll try to explain it to you.______
Ex. 4. Give detailed answers to the following questions: 1. What can you say about water in nature? 2. What is the composition of watcrf 3. What properties make it a unique liquid? 4. What is the structure of water interesting for?
Ex. 5. Discuss the following topics:
1. The Physical Properties of Water.
2. The Structure of Water.
3. Water as a Universal Solvent.
WHAT IS IT?
A part of a solution which is present in greater amount.
Part Four THE ATTRIBUTE
Lesson 25
Scction I Ex. 1. Practise your reading. The subject of acids and bases led to the development of an interesting scries of theories.Text 25 A Bases
In the 17th century, during the infancy of experimental chcmistry, acids and bases were defined or described on the basis of dicir behaviour. Tlius,… In the 18th century, following the discovery of oxygen by Joseph Priestley,… These objections led to more or less conflicting theories: the protonic theory advanced by Bronstcd and Lowry in 1923…Text 25 В
Прочтите текст про себя (контрольное время чтения — 3 минуты).
The Arrhenius Theory of Acids and Bases
of chcmical changcs, so that the principles and practical points arc ofvery general use. Now wc must devote some time to a discussion of… The classification of substanccs as acids was at first suggested by their sour… An explanation of why acids had differing strengths was one of the important results of the Arrhcnius ionic…Lesson 26
Section I Ex. 1. Practise your reading. Liquid solutions provide an extremely convenient means of bringing together carcfully measured amounts of reagents and…Text 26 A Liquids and Solutions
One of the most important properties of liquids is their ability of acting as solvents. In the first place, liquid solutions provide an extremely… One of the most engaging and absorbing features of the study of chcmistry is… Words and Word-Combinations to Be MemorizedLesson 27
Section I Ex. 1. Practise your reading. It is often true that the most common concepts we use arc the most difficult to define precisely.Text 27 A The Properties of Solutions
mixtures. A solution of soap in water has a cloudy appcarancc due to particles which consist of many soap molcculcs collected together. Such a… The requirement that solutions have continuously variable composition… Words and Word-Combinations to Be MemorizedText 27 В
Just as the variables, pressure, volume, and temperature, were used to describe the state or condition of pure gases, liquids, and solids, these and… There arc many possible types of solute-solvent pairs to be listed later. A… 1) liquid in liquid; 4) liquid in solid;Part Five THE ADVERBIAL MODIFIER
Lesson 28
Section I Ex. 1. Practise your reading. As long ago as 1833, it was concludcd that electrolysis took place through the transport of electricity by mobile…Interactions in Electrolyte Solutions
A good deal of success in the study of molcculcs in the gas phase prompted chemists to attempt to build up theories of solutions in an analogous… As long ago as 1833, both Faraday and Danicll concluded that electrolysis took… Words and Word-Combinations to Be MemorizedText 28 В
About the mid-1880's, Arrhcnius postulated in his ionization theory that (1) electrolytes arc completely dissociated into their constituent ions in… The first statistical theory of electrolyte solutions, the intcrionic… dissociated into ions, and observed deviations from this ideal behaviour arc then ascribcd to electrical interactions…Lesson 29
Section I Ex. 1. Practise your reading. The temperature remaining constant, the fraction of liquid molcculcs with enough kinetic energy to evaporate remains…Text 29 A Liquid-Vapour Equilibrium
Now, let us analyse what happens when a liquid is placed in a closed evacuated container. Immediately, the liquid starts to evaporate at a rate… The time dependence of the evaporation and condensation rates is worth… Words and Word-Combinations to Be MemorizedAnalyse, attractive, bind, constant, dependence, initial, magnitude, note, primarily, proportional, situation, spontaneous, substantial, it was not until... that, not until, vessel, zero
Ex. 5. Give the Russian equivalents for the following:
evaporate, entirely, overcome the forces, leave the liquid, the magnitude of the energy, range from... to, substantial, overcome the binding forces, be proportional to, remain the same, start immediately, the dependence is worth considering, become equal to, per unit time, consequently, at a fixed temperature, remain unchanged, opposing processes
Ex. 6. Give the English equivalents for the following:
относительно низкая температура, поместить в сосуд, преодолеть силы притяжения, самопроизвольно, средняя потенциальная энергия, открытый сосуд, давайте проанализируем, начинать испаряться, пер. воначальио, с постоянной скоростью, давление пара растет, положение равновесия
Ex. 7. Fill in the blanks with articles where necessary.
1. Let us analyse what happens when ... liquid is placed in ... closcd evacuated container. 2. As long as ... temperature remains constant, ... evaporation continues at... constant rate. 3. As ... pressure of... vapour grows,... number of... gas molcculcs returning to... liquid also increases.
4__ time dependence of... evaporation and condensation rates should be
considered. 5. At equilibrium,... evaporation and condensation<lo not stop.
Ex. 8. Give synonyms for the following:
magnitude, mention, substantial, fraction, remain, placc, primarily, initially, increase, equal
Ex. 9. Give antonyms for the following:
low, open, leave, minimum, the same, continue, container, immediately, start, also, consequently
Ex. 10. Translate the sentences into Russian.
1. Heating the substancc, one must be very attentive. 2. While moving, molccules collidc with cach other. 3. When at the laboratory, one must observe safety rules. 4. Our lecturer being ill, wc had no lecture yesterday. 5. Our assumption confirmed, wc could continue the experiment. 6. The lccturc being over, wc shall have a long break for dinner. 7. If present in air in larger amounts than 1 in 20,000 by volume, ozone is irritant and poisonous. 8. Practically, all acids when pure arc polar molecular structures. 9. Having made a number of experiments with calcium and sulphuric acid at the temperature of 40°C, the Russian engineer Pctrov was the first to put forward the problem of chcmical activation. 10. One must be very careful when heating potassium chloratc. 11. Any element when combining with oxygen forms an oxide. 12. While dealing with chcmicals in a laboratory, one can't do without such apparatus as funnels, beakers and so on. 13. Oncc discovered, the periodic system of the elements received much scientific attention. 14. Unless otherwise stated, volumes of gases always refer to standard conditions of temperature and pressure. IS. No substancc can be considered chemically dry unless specially treated. 16. Large pieces of sodium may producc dangerous explosions if placcd
0n water. 17. Sodium hydroxide is prepared industrially by two general jncthods, the oldest being the reaction of sodium carbonate and calcium hydroxide. 18. Hydrogen peroxide being added to an acidified solution of potassium permanganate, bubbles of gas arc evolved, the gas evolved being oxygen. 19. The formula of a compound being known, wc can calculate its molccular weight. 20. The liquid state being intermediate between the solid and gaseous state, die properties of the liquids show similarities to those of both solids and gases. 21. Cobalt and nickel arc much more resistant to atmospheric oxidation than iron, with nickel being especially resistant. 22. Considered from this point of view, the reaction mechanism seems to depend only on the following three factors. 23. Having analysed the data, the author found that they were in agreement with the theory. 24. Having been heated to 100°C, water began to boil. 25. Having finished the experiment, we must proccss the data. 26. Except where otherwise stated, the measurements were taken at room temperature. 27. Other things being equal, pressure grows proportionately to the temperature. 28. Having discovered the law of periodicity of the chcmical elements, Mcndclcycv made his greatest contribution to the development of chcmistry. 29. Studying the properties of any substancc, the chcmist has to perform a number of experiments. 30. Lavoisier (1745-1794), believing oxygen to be a constituent of all acids, gave it the name oxygen (Greek: acid-former).
Ex. 11. Translate the sentences into English without using a dictionary.
1. Если жидкость с низкой температурой кипения находится в открытом сосуде, она в конце концов полностью испарится. 2. Молекулы жидкости связаны с соседними молекулами силами притяжения. 3. Некоторые молекулы обладают достаточной кинетической энергией для того, чтобы преодолеть силы связывания и перейти в пар. 4. При прочих равных условиях, пока температура остается постоянной, испарение продолжается с постоянной скоростью. 5. Если жидкость находится в закрытом сосуде, необходимо рассмотреть процесс конденсации.
Ex. 12. Answer the following questions:
1. What happens with a liquid if it is placed in an open container? 2. What forces act between the molecules in a liquid? 3. Under what conditions can some molcculcs leave the liquid? 4. What happens if a liquid is placed in a closed evacuated container? 5. What docs the rate of condensation depend upon? 6. What situation is callcd equilibrium between the liquid and vapour phases?
Scction II
Унр. 1. Назовите значения следующих интернациональных слов:
normal, initiation, guarantee, final, introduce, agent, porous, ceramic
? Степаном I 93
Упр. 2. Проверьте, помните ли вы значения следующих слов; если нет, обратитесь к словарю:
sensitive, raise, bubble, exert, hence, reach, violence, exceed, avoid, evolve
Text 29 В
Прочтите текст про себя (контрольное время чтения—3,5 минуты).
Temperature Dependence of Vapour Equilibrium
The initiation of a bubble in the bulk of a pure liquid is a very difficult proccss, sincc it requires that many molcculcs with kinctic energies… Упр. 3. Передайте основное содержание текста в нескольких предложениях. Упр. 4. Какие слова в тексте означают «в свою очередь»?Lesson 30
Section I Ex. 1. Practise your reading. It is a matter of common experience that the capacity of a solvent to dissolve in a given solute is often limited.Text 30 A Solubility
The liquids thai form an ideal solution arc always miscible in any proportions and, thus, have infinite solubility in cach other. The reason for… Words and Word-Combinations to Be Memorized as is the case with, as the case may be, in case, in which case, it is far from the case, it is not the ease with,…Text 30 В
Прочтите текст про себя (контрольное время чтения —3,5 минуты).
Nonideal Solutions
solid solute —» liquid solute —» solute in solution. The second of these steps docs not involve any energy change, for the solution… By using some carc, wc can extend our aigumcnts to nonideal solutions. Two liquids which mix with the evolution of…Part Six PARENTHESES
Lesson 31
Section I Ex. 1. Practise your reading. The terms "oxidation" and "reduction*' now arc applied to reactions in which neither oxygen nor…Text 31 A Oxidation and Reduction
removal of oxygen. Needless to say, hydrogen seems to be the chemical opposite of oxygen (the two elements combine readily, and arc evolved at… 2S02 + 02« 2SOj (oxidation of S02; addition of oxygen) PbO + H2 — Pb + H20 (reduction of PbO; removal of oxygen)Agent, e. g., evolve, generally speaking, loss, meaning, monoxide, needless to say, neither... nor, non-metal, opposite, permanganate, peroxide, put into operation, put into pracitcc, put it (in) another way, put off, removal, remove, say nothing of, seem, summarize
Ex. 5. Give the Russian equivalents for the following:
the meaning of the term, needless to say, combine readily, a fuller meaning, add to, remove from, apply to, take an example, obviously, regard as, be the opposite, bring about oxidation, involve a change, by gain of electrons, by loss of electrons, acccpt, supply
Ex. 6. Give the English equivalents for the following:
вообще говоря, легко соединяться, выделяться на электроде, быть похожим на, следовательно, применять к реакции, ни... ни..., например, подобным образом, заметим что, увеличивать валентность, иными словами, полное определение, азотная кислота, сульфид водорода, аммиак, большинство неметаллов, как уже упоминалось, удаление электронов, окислитсль, восстановитель
Ex. 7. Fill in the blanks with articles where ncccssarv.
1. ... oxidation is ... removal of... electrons from ... substancc. 2.... oxidation of... chlorine to... chloride ions takes placc by... gain of ... electrons. 3. ... oxygen is ... oxidizing agent. 4. ... meanings of... terms ... oxidation and ... reduction arc considered here. 5.... change of ... ferrous oxide to ... ferric oxide is obviously ... oxidation.
Ex. 8. Give synonyms for the following:
addition, readily, therefore, full, apply, take an example, changc, obviously, regard, includc, occur, take, give
Ex. 9. Give antonyms for the following:
complex, removal, opposite, decompose, reduction, dccrcasc, positive, metal, incomplete, exclude, loss, acccpt
Ex. 10. Translate the sentences into Russian.
1. To anticipate a little, there are several meanings of the terms "oxidation" and "reduction". 2. To be sure, any student can easily give an example of oxidation. 3. To begin with, one can say diat the simplest meaning of the term "oxidation" is the addition of oxygen to a substancc and reduction is the opposite. 4. Needless to say, this meaning is incomplete. 5. To sum up, relatively little is known about the state of solid solutions or the conditions of equilibria which exist therein. 6. To mention only one, we shall consider in some detail the theory of electrolytic dissociation in its application to homogeneous equilibria. 7. Suffice it to say that similar results can be derived for other systems under investigation. 8. To put it in another way, the attraction between molcculcs varies inversely. 9. To tell the truth, these measurements vary so wridcly that it seems difficult to include them in this article. 10. Thorium, plutonium, mcndclcvium — not to mention uranium — belong to the actinoid scries. 11. To summarize, no satisfactory equation for the proccss has been proposed. 12. The method is somewhat risky and not easily generalized, to say the least. 13. To put it more cxactly, these values arc now regarded as normal. 14. To say nothing of the details, an oxidizing agent is a substancc which brings about oxidation. IS. Metals and hydrogen form positive ions, that is to say, they arc clcctropositivc. 16. To take an example, hydrogen and carbon arc reducing agents. 17. To illustrate, a change of ferrous ion to fcrric takes placc by loss of an electron. 18. One of the aims of education is to extend student's views, his philosophy, so to say. 19. As emphasized above, this condition is satisfied automatically. 20. As already mentioned, we find cases where solution occurs with evolution of heat. 21. Roughly speaking, the conccption of free ions affords very satisfactory explanation of all these phenomena. 22. Broadly considered, a heterogeneous system is one which consists of more than one physical state. 23. Put another way, one molecule gives rise to two smaller molecules or atoms. 24. Strictly speaking, it is for this reason that we shall to a large extent limit ourselves to a brief consideration of a gaseous state. 25. As pointed out previously, the term "oxidation" has a long history. 26. As stated above, the meaning of reduction is the opposite of oxidation. 27. Generally speaking, oxidation is removal of electrons.
Ex. 11. Translate the sentences into English without using a dictionary.
1. Первое, о чем обычно думают, говоря об окислении, — это то, что окисление представляет собой присоединение кислорода. 2. Соответственно, восстановление — это противоположный процесс. 3. Однако теперь эти термины применяются и к реакциям, в которых ни кислород, ни водород не участвуют. 4. Современные понятия окисления и восстановления связаны с переносом электронов. 5. Для химика окисление — это удаление электронов.
Ex. 12. Answer the following questions:
1. What is the meaning of the term "oxidation"? 2. In what sense is hydrogen the chemical opposite to oxygen? 3. Why is the changc of any ferrous compound to a ferric compound regarded as an oxidation? 4. What is an oxidizing agent? 5. What is a reducing agent? 6. What arc the most common oxidizing and reducing agents?
Scction II
Упр. 1. Назовите значения следующих интернациональных слов:
sort, extensively, systematically, introduction, primitive, metallurgical, restoration, nomenclature, term, natural, original
Упр. 2. Проверьте, помните ли вы значения следующих слов; если нет, обратитесь к словарю:
prior to, combustion, phlogiston, conccivc, charcoal, dual, rust
Text 31 В
Prior to the discovery of oxygen independently by Scheclc of Sweden in 1771-1772 and by Joseph Priestley of England in 1774, combustion had been… Lavoisier called the product formed by addition of oxygen an oxide. Therefore,… To tell die truth, long before the introduction of the term "oxidation" the term "reduction" had…Part Seven EMPHATIC CONSTRUCTIONS
Lesson 32
Scction I Ex. 1. Practise your reading. It was largely analytical chemistry that existed in the 18th and most of the 19th century.Text 32 A
Analytical Chemistry — the Oldest Field of Chemistry
The first problem to engage the interest of most chcmists was to determine as exactly as possible the composition of the earth. Greater emphasis… It was in the latter half of the 19th ccntury that the so-called… The pressing and dyeing of textiles, the production of glass, leather, soap — these arc merely four examples (many…Area, analytical, as... as (possible), broad, chief, chiefly, consume, demand, etc., exactly, be forced, former, highly, house, indeed, justification, laner, the former... the latter, literature, by/in nature, not at all, operation, perhaps, press, quote, raw, in a sense, in the sense of, stage, succcssful, successfully, title, traditional, traditionally, usefulness, view, yet
Ex* 5. Give the Russian equivalents for the following:
the phlogiston theory, demonstrate the fallacy of a theory, in a very particular sense, indeed, with justification, place emphasis on, because of one's views, be more of a scientist than, in the evolution of the natural sciences, the so-called "industrial revolution", raw materials, the production of glass, quote an example, slowly but surely, modest changes, be inorganic in nature
Ex. 6. Give the English equivalents for the following:
аналитическая химия, требовать, блестящий химик, современная химия, называть отцом химии, экспериментальная работа, истинный химик, отличаться от, быть вынужденным, как можно точнее, состав вещества, на этой стадии, вклад ученых, быть чрезвычайно важным, служить основой, не будет преувеличением сказать, в то время, во второй половине XIX века, быть скромным, промышленное производство, состоять из, за исключением природных красителей
Ex. 7. Fill in the blanks with articles where necessary.
1. Analytical chcmistry is regarded as ... oldest field of... chcmistry.
2. Many years were needed to demonstrate... fallacy of... phlogiston theoiy.
3. Avogadro's law is extremely important in... chemistry of... gases. 4.... pressing and dyeing of textiles are ... examples of operations that moved into ... factory. 5. Most of... natural dyes were inorganic in ... nature.
Ex. 8. Give synonyms for the following:
branch, wide, require, show, remarkable, accurately, step, however, highly, mainly, occur, production
Ex. 9. Give antonyms for the following:
young, ncccssary, from, more, impossible, usefulness, slowly, successful a considerable number
Ex. 10. Translate the sentences into Russian.
1. It is the analytical chcmistry that is regarded as the oldest field of chcmistry. 2. It is M. V. Lomonosov who is the founder of Russian physics and chcmistry. 3. It was my supervisor who advised me to use this apparatus.
4. It was Mendcleyev's periodic law which served as a key to discovering
new elements. 5. It was not my teacher whom I addressed my question to. 6. It was in 1869 that Mcndeleycv's periodic system was published. 7. It is horizontal rows of the periodic table which arc callcd periods. 8. Ozone does remove harmful ultraviolet radiation from sunlight. 9. It is not this examination that is the most difficult this term. 10. It was not till late in the 19th ccntury that numerous household items began to be produced at factories. 11. These results do support the kinetic treatment of the behaviour of particles in colloidal solutions. 12. It is only at ordinary temperature that the agreement between the two methods is satisfactory. 13. It was not until the results conccrning solid solutions had been obtained that a general conclusion was reached. 14. The reaction docs procccd slowly in most cases. IS. It was evident that the resulting mixture did obey the mixture law. 16. The use of deductive methods shows that all these cases come from one and the same root. 17. It is not until a substance undergoes distribution that it has the same molecular weight in the two phases. 18. It is not until two pieces of zinc and coppcr are brought into contact that they become electrified. 19. In the actual case the density of the vapour does alter with the height. 20. It was not until oxygen was discovered that many processes could be understood.
Ex. 11. Translate the sentences into English without using a dictionary.
1. Считается, что аналитическая химия — старейшая отрасль химии. 2. Потребовалось много лет, чтобы показать, что теория флогистона неверна. 3. Развитие экспериментальных методов внесло большой вклад в исследование состава веществ. 4. Развитие промышленности сыграло большую роль в разработке новых методов аналитической химии. 5. Многие операции, производившиеся ранее дома, стали производиться в промышленных масштабах.
Ex. 12. Answer the following questions:
1. Whose investigations helped to prove the fallacy of the phlogiston theory? 2. Why is Lavoisier callcd the "father" of analytical chcmistry?
3. Why is analytical chemistry regarded as the oldest branch of chcmistry?
4. What problems engaged the interest of most chemists at that time?
5. Why did the development of industry stimulate the development of analytical chcmistry?
Section II
Упр. 1. Назовите значения следующих интернациональных слов:
process, routine, test, prestige, technician, person, professional, era, indicate, apparatus, finish, front, organization
Упр. 2. Проверьте, помните ли вы значения следующих слов; если нет, обралггесь к словарю:
plant, employ, quality, quantitative, constituent'perform, be concerned with, exclusively, relationship
Text 32 В
Прочтите текст про себя (контрольное время чтения — 2 минуты).
Two Branches of Analytical Chemistry
The "works chcmist" or analyst of this era was concerned almost exclusively with two branches of analytical chemistry, namely, qualitative… It is these two branches of chemistry that are still regarded to be main… Упр. 3. Передайте основное содержание текста в нескольких предложениях.Lesson 33
Section I Ex. 1. Practise your reading. Gradually, chemists began synthesizing some things found in nature and later those not found in the natural state.Text 33 A Classical Methods of Analysis
Difficult as the situation of the so-called "works chcmist" during the industrial revolution was, it was not the sole reason for the… Following the logical sequence of events, it is not at all strange that… About halfway between World War I and World War II, the analytical chcmist gradually began to experience a renaissance…Text 33 В Modern Methods of Analysis
The so-called classical gravimetric and volumctric methods have by no means been superseded by physical chcmistry and physical methods. Unlikely as… One of the more modern developments in the field of analysis is that of… Today the research analyst in the chcmical proccss industries is an honoured member of the "team". The use…Lesson 34
Scction I Ex. 1. Practise your reading. Used in the design and interpretation of chcmical experimentation are various statistical methods.Statistical Methods in Analytical Chemistry
In taking cognizance of the unavoidable experimental errors, rather than in ignoring them or dismissing them as negligible, and in attempting a… Words and Word-Combinations to Be Memorized aid, by-product, common sense, construct, construction, contribution, correction, criterion, data, design, error,…Lesson 35
Scction I Ex. 1. Practise your reading. When a solid is heated to incandescence, it emits more or less continuous spectrum.Text 35 A Investigations of Spectra
When a solid is heated to incandcsccncc, it emits a more or less continuous spcctrum, but gases and vapours under the same conditions, when examined… A line spectrum is the one formed when radiation from an incandescent gas is… In a molecular spcctrum, a scries of fairly broad bands is formed. These bands arc sharp at one edge but dying away on…Text 35 В
The more detailed is the scrutiny of the history of chcmistry, the greater is the number of interesting facts that come to light. Those accustomed to associate the name of Kirchhoff with the discovery of… He pointed out that the method could be used to determine the composition of celestial bodies, thus laying the…Lesson 36
Section I Ex. 1. Practise your reading. The guiding light of theory must be confirmed by the bench of physical manipulation.Text 36 A Choosing Chemistry' a Profession
The answer for the student who intends to major in a science is obvious. His hope is to derive information to use as a tool in making a living. There is an answer, however, beyond the fact that the coursc may be… The period of the teens and early twenties is a crucial one in life. It is during this time that the student is…Text 36 В
Прочтите текст про себя (контрольное время чтения — 3 минуты).
Why Study Chemistry?
It is easy to attack any specific course as contributing little or nothing that the student will use after graduation. However, the huge reality and… It is not wise to press the analogy too far. Beside this complex of attitudes… But what of that information which has been lost unused, is other than scaffolding? The answer is that it has not been…TRANSLATION PRACTICE Texts
Text 1
Conductance and Electrolysis
If a strong clcctrolytc is formed as a result of a chcmical reaction involving two weak clcctrolytcs, the conductancc of the resulting solution… Electrolysis always accompanies the passage of a dircct current through an…Text 2 Library and You
helping him to accomplish this in an informal fashion is to encouragc him to acquire for his own collection a corc of reference books. A great…Text 3 Infrared Spectroscopy
The techniques of Raman and IR spectroscopy arc generally considered complementary in the gas and solid phases bccausc some of the spccics under… Text 4Nuclear Magnetic Resonance
Were this NMR to depend only on the nuclei of the species present in the solution, the technique would be without point for the identification of… (о provide information on the type of association between an ion and its…Text 5 Gold
Why? Well, wc can supply three reasons. Value. Beauty. Permanence. Obviously, there… Dcspitcour glowing words above, metallic gold has very few practical uses. It is really a metal to be looked at, not…Text 6 Actinium
Actinium is exclusively tripositivc and resembles the tripositivc rare earth elements in its chcmical properties. It forms insoluble compounds of… Except for the sulphide, the compounds of actinium arc colourless. All of the… Text 7Radiation Effects on Polymers
The cross-linking of polymers by radiation has been much studied. The irradiation of any organic compounds results in breaking of CH bonds, leaving… Whatever the mechanism of cross-linking may be, the result is of commercial… Text 8Л Metal that Doesn't Sink
Silver-white magnesium is lighter than aluminium and superior to it in heat capacity and in its capability to act as the main component of various… As for lithium, the third clement on the Mcndclcycv periodic table, it is the… According to expert opinion, there is much more lithium in the earth's crust dian, say, zinc or tin, 130 times more…Text 9
Insulator Itirns into Superconductor
Superconductivity, at which a conductor completely lacks resistance to elcctric currcnt, was discovered more than 70 years ago. This phenomenon… Present-day clcctronic, clcctrotcchnical apparatuses, instruments and machines… conditions of low temperatures. Among Uicm are radio-receiving devices for dctccting weak signals arriving from the…Text 10 Salt Shaker Wedding
The bride was given in marriage by her uncle, Mr. Argon Inert, one of the community's most prominent bachelors. The bride's eldest sister Miss… Mr. Alkali chose his best man his brother, Mr. Potassium Alkali. Mcsscrs… Following the ccrcmony, a reception was held at the Electrolytic Tea Room for the immediate family. Brine and…Text 11
The Role of Theory in Chemistry
How diverse is it? How accurate is it? How simple is it?Theories of Matter
Quantum theory has two essentially equivalent versions: one concerned with wave mechanics (derived from Schrocdingcr's work) and one concerned with… The numerical and algebraic calculations required in the MMTM procedure can…Molecular Theory
An example of a semiempirical molccular theory is the n-electron theory of conjugated, unsaturated hydrocarbons. Here the size of the vcctor space…Text 14
Differentiating between Primary, Secondary, and Tertiary Alcohols
A convenient procedure is as follows: A 4-inch test-tube is fitted with a onc-holc rubber stopper carrying a glass rod which rcachcs to the bottom of the test-tube. Glacial acetic acid (3 ml.) is introduced into die… If no dccolourization of the KMn04 takes placc, the alcohol is tertiary. If dccolourization takes placc (best…Text 15
A Brief History of Polypeptide Chemistry
It was not the early work on protein chcmistry, however, that led to the conccpt of enzymes as catalysts of biological reactions. Indeed, it was the… In 1926, Sumner made a tremendous advance when he recognized that enzymes…Text 16
Characteristics of Mossbauer Spectra
A typical Mossbauer spcctrum is a characteristic plot of the total number of events (counts) observed as a function of sourcc-absorbcr velocity (the… The profile of the resonance maximum obeys (ideally) the Lorcntz… constant (£-£<>) + /4 r2Text 17 Free Radicals
One way of making the methyl radical as a dilute gas is by heating mercury dimethyl, Hg(CH3):, which decomposes to give metallic mercury and methyl… The American chcmist Moses Gombcrg (1866-1947) discovered in 1900 that some…Text 18
The Manufacture of Sulphuric Acid
In the contact proccss sulphur trioxidc is made by the catalytic oxidation of sulphur dioxide (the name of the proccss refers to the fact that… In the lcad-chambcr proccss oxygen, sulphur dioxide, nitric oxide, and a small… 2SO:+ NO + NO:+ 0:+ H2O -> 2NOHSO4;What Is Light? What Is an Electron?
These questions cannot be answered by one of the two stated alternatives. Light is the name that wc have given to a part of nature. The name refers… Some of the properties of light resemble those of waves, and can be described… In the same way, an electron is neither a particle nor a wave, in the ordinary sense. In many ways, the behaviour of…Text 20 The Nature of Resonance
The goal of a structure investigation of a system is the description of the system in terms of simpler entities. This description may be divided…Text 21
Benjamin Franklin and Electricity
Kant once remarked that Benjamin Franklin was a new Prometheus who had stolen fire from heaven. In his own day, Franklin was celebrated throughout all Europe as the world's foremost electrician and his book on the… Franklin saw his first clcctrical demonstration in Boston in 1746. He purchased all the apparatus used by the British…Text 22 Future Perspectives
point is cspccially important when wc remember that "a food is not a food until it is eaten", and it is necessary that someone be willing… In addition to protein by fermentation, one can make specific products like… There will continue to be a need to trap our widespread but difficult- to-usc resources such as coal and oil shale,…Text 23
Gas Chromatography Methods
In rcccnl years, the versatility of GC has been greatly extended by the so-called ancillary techniques. This refers to the coupling of different…Text 24
Liquid Chromatography Detectors
High resolution column LC is a technique which is experimentally analogous to GC, in that one makes use of small sample sizes (microlitrc… The advantage of liquid chromatography is that thermally unstable, nonvolatile… The current interest in column LC is evidenced by numerous articles which arc now appearing in the literature. Column…Miscellaneous Grammar
1. The work referred to also brought to light many examples of abnormal behaviour. 2. Having examined it carefully, wc found out that the gas under… 22. Applying the law of mass action, the following equation was obtained. 23. The values given below arc calculated on the assumption that 1 gram- molecule of the substancc under examination…Appendices
I
Words a Student Should Know before Studying the Textbook
a[10] about* above active after afternoonD
daily dark day
December 9 Степаном
decide
defend
democracy
democratic
demonstrate
describe
desk
die
different
difficult
dinner
dirty
discover
do* (did*) doctor dog door down* dress drink during duty
E
each
early
east
easy
eat
economic
economy
eight
eighty
eighteen
eighth
eleven
eleventh
else
empty
end
England
English
enough
enter
evening
in the evening every everybody everyone everything
explain exploit eye
F
face factory family far
farmer father Februaiy field fifteen fifth fifty fight film find finish fire first* fish five flag food football for* forest forget forty four fourteen fourth Friday friend friendly from* from Moscow from 5 to 7 o'clock front
in front of full
G
game
garden
get*
get acquainted with get up girl
give glass
go* (went*) go away go on go out go to bed good (better, best) grammar grass great green grey ground grow
H
half
hand
happen
happy
hard
hat
have* (has*, had*) have dinner (supper, breakfast, tea...) have a nice time have to he* hear heart heavy help her* here* high him* his* history hockey holidays home
at home homework hope hot how how long how many how much
hundred hungry
I*
it* in*
industrial
industry
interesting
international
into*
invite
it*
its
January
job
June
July
just*
К
kill
king
know
lake land
language
large
last
late
learn
leave
left
lesson
let
let's (let us) letter life light like* listen little* live long look*
look out
loud love low
M
make* man (men)
many* (more*, most*)
March
march
May
may*
me*
mean
meat
meet
meeting
member
middle
milk
million
mine
minute
mistake
Monday
money
moon
morning
in the morning mother mountain move much* museum music must* my*
N
name What is your name? — My name is... national near
necessary need never new*
newspaper next
night
at night nine nineteen ninety ninth no*
nobody
nothing
noon
north
not*
notebook November now* number
October of
the book of my friend ofP often old* on* on Monday on the table once one* only* open or*
organize other* our* out*
out of over* be over
page
parents
park
part
party
pay
peace
pen
pencil
people
picturc
place
plan
plane
play
pleasant
political
poor
port
prepare
pronounce
pupil
put
quarter
question
quick
quickly
quiet
rain
ratio
read
receive
red
remain remember rest rich
right* (2)
rise
river
road
room
round
run
Russia Russian
Saturday say* (said*) school sea
| September seven seventeen seventh seventy shall share she* ship shoe shop short show side simple simply sister sit sit down six sixteen sixth sixty ski sky sleep slow slowly small snow so* society soft some* somebody something south speak spend sports spring in spring square stand stand up star start stop story street |
second see* sell send
Strong
Struggle
study suit summerChemical Elements
IllWord-building
Образование одного слова из другого производится следующими способами. 1. Без изменения произношения и написания слова: у существительных и… attack—атака to attack — атаковатьОбщие схемы образования производных слов
{ Г" anion cation I i ionPrepositions of Place and Direction
above
over

|
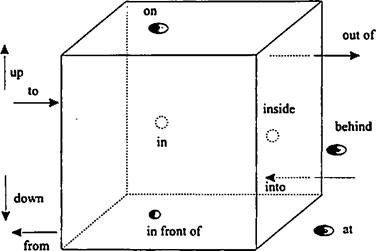 under
under
|
с
V
Irregular Verbs
БИБЛИОТЕКА ВГУ
Содержание
Предисловие................................................................................. 3
Part One. The Predicate.................................................................. 4
Lesson 1. Определение сказуемого в предложении. Простое сказуемое в действительном залоге (Indefinite). Употребление глагола willwvi выражения регулярности действия в настоящем, глаголов used to и would для выражения повторности
в прошлом. Функции глагола do................................ 4
Text 1 A. Hypotheses, Theories and Laws............................ 5
Text 1 B. Dalton's Atomic Theory......................................... 9
Lesson 2. Простое сказуемое в действительном залоге (Continuous,
Perfect)........................................................................ 11
Text 2 A. The World's Greatest Chemist.............................. 12
Text 2 B. The Mendeleyev Story......................................... 16
Lesson 3. Простое сказуемое в страдательном залоге 18
Text 3 A. The History of the Periodic Table........................ 19
Text 3 B. The Periodic Table of the Elements..................... 23
Lesson 4. Особые случаи выражения сказуемого глаголом
в страдательном залоге............................................. 25
Text 4 A. Oxygen: History and Occurrence......................... 26
Text 4 В. Oxygen................................................................ 30
Lesson 5. Согласование времен................................ 32
Text 5 A. Modem Uses of Oxygen...................................... 34
Text 5 B. Ozone: Properties, Toxicity, and Applications..... 38
Lesson 6. Составное именное сказуемое. Общие сведения о неличных формах глагола. Способы выражения предикатива............................................ 40
Text 6 A. Hydrogen............................................................. 41
Text 6 В. Hydrogen Production........................................... 46
Lesson 7. Составное именное сказуемое. Типы глаголов-связок 48
Text 7 A. Silicon Dioxide.................................................... 49
Text 7 В. Silicon: an Essential Element for Life Processes.. 53
Lesson 8. Составное глагольное сказуемое. Формы инфинитива и их
значения. Модальные глаголы и их заместители... 55
Text 8 A. Selenium.............................................................. 56
Text 8 В. Selenium Compounds.......................................... 61
Lesson 9. Выражение вероятности действия при помощи модальных
глаголов.................................................................... 63
Text 9 A. The Halogens....................................................... 64
Text 9 В. Fluorine............................................................... 68
Lesson 10. Формы и употребление сослагательного наклонения 70
Text 10 A. Indium............................................................... 72
Text 10 В. Uses of Indium.................................................. 75
Lesson 11. Употребление н перевод глаголов тау might, could,
ought в сослагательном наклонении....................... 77
Text 11 A. Fascinating Phosphorus..................................... 79
Text 11 В. Modifications of Phosphorus............................. 82
Lesson 12. Повторение темы «Сказуемое»............................ 84
Text 12 A. Chemical Symbols for Elements........................ 85
Text 12 B. Chemical Symbols for Representing Compounds 89
Part Two. The Subject............................................................... 92
Lesson 13. Подлежащее. Существггтельное в функции подлежащего.
Местоимение в функции подлежащего.................. 92
Text 13 A. The History of Chemistry.................................. 93
Text 13 В. New Frontiers in Chemistry............................... 97
Lesson 14. Оборот «именительный падеж с инфинитивом». 99
Text 14 A. The Idea of the Atom...................................... 100
Text 14 B. The Atomic Theory of Dcmocritus and Dalton 103
Lesson 15. Некоторые особенности перевода на русский язык
оборота «именительный падеж с инфинитивом». Оборот «именительный падеж с причастием, прилагательным» числительным»................. 105
Text 15 A. The Atomic Theory.......................................... 106
Text 15 B. The Bohr Theory of the Hydrogen Atom......... 109
Lesson 16. Инфинитив и инфинитивный оборот в функции
подлежащего.......................................................... 111
Text 16 A. Molcculcs........................................................ 112
Text 16 В. Molecular Composition and Size...................... 115
Lesson 17. Герундий и герундиальный оборот в функции
подлежащего.......................................................... 117
Text 17 A. Molecules in Gases and Liquids....................... 118
Text 17 B. Molecules in Solids.......................................... 121
Lesson 18. Придаточное предложение в функции подлежащего 123
Text 18 A. The Nature of a Liquid.................................... 124
Text 18 B. The Nature of a Gas......................................... 127
Part Three. The Object..................................................................... 130
Lesson 19. Место дополнения в предложении. Существительное
и местоимение в функции дополнения................. 130
Text 19 A. The Study of the Structure of Molecules.......... 131
Text 19 B. The Development of the Theory of Valcnce.... 134
Lesson 20. Инфинитив и инфинитивный оборот в функции
дополнения............................................................. 136
Text 20 A. The Structure of Covalcnt Compounds............ 137
Text 20 B. Covalcncc........................................................ 141
Lesson 21. Причастный оборот в функции дополнения...... 143
Text 21 A. Factors Influencing the Rate of Reactions........ 144
Text 21 B. A Catalyst........................................................ 147
Lesson 22. Герундий и герундиальный оборот в функции
дополнения............................................................. 148
Text 22 A. Factors Affecting the Boiling Point.................. 149
Text 22 B. Temperature Scales.......................................... 152
Lesson 23. Придаточное предложение в функции дополнения 154
Text 23 A. Celsius versus Centigrade................................ 155
Text 23 В. The Kelvin Temperature Scalc and Modem Means
of Measuring the Temperature............................ 159
Lesson 24. Повторение тем «Подлежащее» и «Дополнение» 161
Text 24 A. The Composition and Structure of Water......... 162
Text 24 B. Water............................................................... 166
Part Four. The Attribute................................................................. 168
Lesson 25. Определение. Прилагательное, местоимение,
существительное, наречие в функции определения 168
Text 25 A. Bases............................................................... 168
Text 25 В. The Arrhenius Theory of Acids and Bases...... 171
Lesson 26. Герундий и герундиальный оборот в функции определения. Причастие и причастный оборот в функции определения. Инфинитив после причастия II и слов likelyу sure, certain 173
Text 26 A. Liquids and Solutions...................................... 174
Text 26 B. A Kinetic Theory of Liquids............................ 177
Lesson 27. Инфинитив н инфинитивный оборот в функции
определения. Придаточное предложение в функции определения 179
Text 27 A. The Properties of Solutions.............................. 179
Text 27 В. Types of Solutions........................................... 182
Part Five. The Adverbial Modifier................................................... 184
Lesson 28. Обстоятельство. Существительное, наречие, герундий и герундиальный оборот в функции обстоятельства...................................... 184
Text 28 A. Interactions in Electrolyte Solutions................. 185
Text 28 В. Ionic Theories.................................................. 188
Lesson 29. Причастие и причастный оборот в функции
обстоятельства. Независимый причастный оборот 190
Text 29 A. Liquid-Vapour Equilibrium............................. 190
Text 29 В. Temperature Dependence of Vapour Equilibrium 194
Lesson 30. Инфинитив и инфинитивный оборот в функции
обстоятельства. Придаточные обстоятельственные предложения 195
Text 30 A. Solubility......................................................... 196
Text 30 В. Nonidcal Solutions........................................... 200
Part Six. Parentheses
Lesson 31. Вводные члены предложения. Инфинитив и причастие
в функции вводного члена предложения.............. 202
Text 31 A. Oxidation and Reduction................................. 202
Text 31 В. Original Meanings of Oxidation and Reduction 206
Part Seven. Emphatic Constructions......................................... 208
Lesson 32. Усилительное do. Эмфатические конструкции
типа It is... that........................................................ 208
Text 32 A. Analytical Chemistry — the Oldest Field of Chemistry ... 209 Text 32 B. Two Branches of Analytical Chemistry.......................................................................... 212
Lesson 33. Эмфатические уступительные предложения...... 213
Text 33 A. Classical Methods of Analysis......................... 214
Text 33 B. Modem Methods of Analysis........................... 217
Lesson 34. Различные случаи инверсии................................. 219
Text 34 A. Statistical Methods in Analytical Chemistry..... 219
Text 34 B. Fundamentals of the Analytical Balancc.......... 222
Lesson 35. Предложения с парным союзом the... the— Двойное
отрицание............................................................... 224
Text 35 A. Investigations of Spectra.................................. 224
Text 35 B. Who is the Discoverer of Spectrum Analysis?. 227
Lesson 36. Общее повторение................................................ 229
Text 36 A. Choosing Chemistry a Profession.................... 229
Text 36 B. Why Study Chemistry?.................................... 232
Translation Practice.................................................................. 235
Texts......................................................................................... 235
Text 1. Conductance and Electrolysis................................ 235
Text 2. Library and You.................................................... 235
Text 3. Infrared Spectroscopy........................................... 236
Text 4. Nuclear Magnetic Resonance................................ 236
Text 5. Gold...................................................................... 237
Text 6. Actinium............................................................... 237
Text 7. Radiation Effects on Polymers.............................. 238
Text 8. A Metal That Doesn't Sink..................................... 239
Text 9. Insulator Turns into Superconductor..................... 239
Text 10. Salt Shaker Wedding........................................... 240
Text 11. The Role of Theory in Chemistiy........................ 241
Text 12. Theories of Matter.............................................. 242
Text 13. Molecular Theory.............................................. ' 243
Text 14. Differentiating between Primary, Secondary, and Tertiary
Alcohols.............................................................. 243
Text 15. A Brief History of Polypeptide Chemistry........... 244
Text 16. Characteristics of Mossbauer Spectra.................. 245
Text 17. Free Radicals....................................................... 246
Text 18. The Manufacture of Sulphuric Acid.................... 246
Text 19. What Is Light? What Is an Electron?.................... 247
Text 20. The Nature of Resonance.................................... 248
Text 21. Benjamin Franklin and Electricity....................... 248
Text 22. Future Perspectives.............................................. 249
Text 23. Gas Chromatography Methods............................ 250
Text 24. Liquid Chromatography Detectors....................... 251
Miscellaneous Grammar.......................................................... 252
Appendices................................................................................. 256
I. Words a Student Should Know before Studying the Textbook 256
II. Chemical Elements........................................................ 261
III. Word-building.............................................................. 264
IV. Prepositions of Place and Direction 275
V. Irregular Verbs 276
Учебное издание
Степанова Татьяна Алексеевна, Ступина Ирина Юрьевна
Английский язык для химических специальностейПрактический курс
English for ChemistsA Practical Coarse
Учебное пособие
Оригинал-макет подготовлен е издательстве Филологического факультета СП6ГУ
Ответственный за выпуск О.С.Капполь Редактор А.И.Миронова Корректор Н.В.Гудкон Верстка: Г. А. Курганова
Изд. № А-1172-1. Подписано в печать 23.11.2005. Формат 60*90/16. Гарнитура «Тайме». Бумага тип. № 2. Печать офсетная. Усл. псч. л. 18,0. Тираж 3000 экз. Заказ № 15811. ^ (jQQ
Издательский центр «Академия)», www.acadcinia-moscow.ru J * Р'
Сатпарно-эпидемиологическое заключение № 77.99.01953Д.004796.07.М от 20.07.2004. 117342, Москва, ул. Бутлерова, 17-Б, к. 360. Тел./факс: (095)330-1092, 3344337.
Лицензия ЛП № 000156 от 27.04.1999.
Филологический факультет Санкт-Петербургского государственного университета. 199034, СПб.. Университетская наб., д. II. Тел./факс: (812)355-0341, 3244)743.
Отпечатано в ОАО «Саратовский полиграфический комбинат». 410004, г. Саратов, ул. Чернышевского, 59.
little — немного, мало; a little — немного употребляются с нснсчислясмымн существительными
few books, little milk, a little water, a few pens, a few tables, little acid, few students, a little coffee
b) Translate the sentences into English.
1. В стакане мало воды. Принеси еще, пожалуйста. 2. Уже поздно, но в лаборатории работают несколько студентов. 3. Только немногие студенты ответили на все вопросы. 4. Вы знаете слишком мало об этом явлении. 5. На столе лежат несколько журналов. В одном из них — статья о Полингс. 6. В вашей работе есть несколько ошибок. Попробуйте их исправить. 7. Студенты нашей группы ссйчас в библиотеке, они занимаются там уже несколько часов. 8. У меня мало денег, я не могу купить этот словарь ссйчас. 9. Несколько студсн-
[1] В скобках указано, сколько вопросов нужно составить.
[2] hard-facing — покрытие твердим слоем, упрочнение поверхности
[3] scant — скудный
[4] beware — остерегаться
As expressed in valence — выраженный валентностью
Has withstood the onslaught — выдержал атаку
All of the atoms of one element being identical — причем всс атомы одного элемента являются идентичными
The several atoms involved — несколько участвующих атомов
[8] unless otherwise stated — если не оговоренб особо
E.s.u. — clcctrostatic unit
Здесь ii далее слова» помеченные звездочкой, следует выучить в первую очередь.
– Конец работы –
Используемые теги: Part, One, The, PREDICATE0.06
Если Вам нужно дополнительный материал на эту тему, или Вы не нашли то, что искали, рекомендуем воспользоваться поиском по нашей базе работ: Part One THE PREDICATE
Что будем делать с полученным материалом:
Если этот материал оказался полезным для Вас, Вы можете сохранить его на свою страничку в социальных сетях:
| Твитнуть |
Хотите получать на электронную почту самые свежие новости?






Новости и инфо для студентов